This website is intended for healthcare professionals only.
Take a look at a selection of our recent media coverage:
15th August 2024
A research team from the Netherlands has found that the stability of monoclonal antibodies (mAbs) is unaffected by the use of drone transportation when compared with traditional car transport for inter-hospital transportation.
They found the stability of mAbs in both vials and infusion bags was adequately maintained during drone transport, indicating that medical drones are a viable and reliable transportation method.
The current reliance on cars to deliver mAbs is compromised by the unpredictable nature of traffic and infrastructure, which leads to unreliable transport times. Drone transport could therefore offer health systems a more efficient and predictable transportation system for mAbs, the authors said.
The researchers assessed the efficacy and safety of medical drone transportation on the stability of mAbs transported in vials and ready-to-administer infusion bags with blinatumomab, tocilizumab and daratumumab. These were Blincyto 38.5 μg concentrate after reconstitution, RoActemra 20 mg/mL concentrate for infusion and Darzalex 20 mg/mL concentrate for infusion, respectively.
These mAbs were chosen as blinatumomab represents a low-concentrate protein drug, tocilizumab represents a drug that may be necessary in emergency settings, and daratumumab is a viable option for home treatment of patients.
Using a VTOL fixed-wing drone, temperature recorders and impact indicators estimated mechanical stress during the flight. Four flights carrying vials and five flights carrying IV bags were performed at a field lab in the Netherlands with an average flight length of eight kilometres and eight minutes of flight time.
Control high-performance size-exclusion chromatography (HP-SEC), dynamic light scattering (DLS), light obscuration (LO), micro-flow imaging (MFI) and nanoparticle tracking analysis (NTA) and absence of visible particles (VI) were employed to assess the presence of aggregates and particle formation in car, drone and control conditions.
Where there was enough quality data, statistical analysis of the vials revealed no significant differences between the control group and the drone-exposed mAbs. Similarly, there were no statistically significant differences between the control, the car and the drone groups for all the infusion bags. By way of example, the data for blinatumomab has been summarised in Table 1 below.
Table 1: Summary of the main results of infusion bag analysis
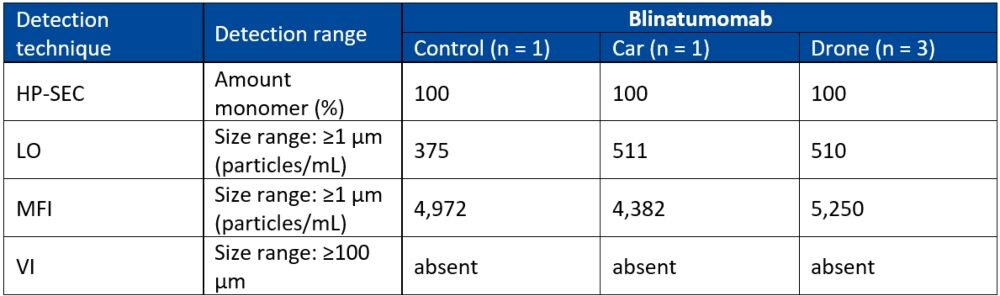
HP-SEC: mean percentage, DLS: mean particle size, LO and MFI: mean total particle concentration, VI: absence of visible particles.
The researchers concluded that integrating drone technology into healthcare logistics has the potential to significantly enhance the efficiency of inter-hospital transportation. Both commercial vials and ready-to-administer infusion bags of mAbs could be transported by drone without resulting in aggregate formation.
Güngören M et al. Investigating the Impact of Drone Transport on the Stability of Monoclonal Antibodies for Inter-Hospital Transportation. Journal of Pharmaceutical Sciences 2024; Apr 03: doi.org/10.1016/j.xphs.2024.04.002.
9th December 2021
RoActemra (tocilizumab) has received approval from the EMA for the treatment of adults with severe COVID-19 and who are in receipt of systemic treatment with corticosteroids and requiring supplemental oxygen or mechanical ventilation.
Until the COVID-19 approval, RoActemra was already approved for use in the management of inflammatory conditions including rheumatoid arthritis, systemic juvenile idiopathic arthritis, juvenile idiopathic polyarthritis, giant cell arteritis and cytokine release syndrome (CRS).
Tocilizumab is a monoclonal antibody that works by inhibiting the binding of interleukin-6 (IL-6) to its receptor and in doing so, Inhibits IL-6 signal transduction of inflammatory mediators in rheumatic diseases. However, emerging evidence has indicated a role for IL-6 signalling in patients hospitalised with severe COVID-19, especially those who are critically ill.
Clinical data
The approval for RoActemra was based on data from three phase III trials. In COVACTA, patients hospitalised with severe COVID-19 pneumonia were randomised, 2:1 to either a single intravenous infusion of tocilizumab (at a dose of 8mg per kilogram of body weight) or placebo. The primary outcome was clinical status at day 28 on an ordinal scale which ranged from 1 (discharged or ready for discharge) to 7 (death). In the published results of the trial, tocilizumab did not result in a significantly better clinical status or lower mortality than placebo at 28 days.
In EMPACTA, hospitalised patients with COVID-19 pneumonia, not receiving mechanical ventilation, were randomised to receive standard care plus one or two doses of either tocilizumab (8 mg per kilogram of body weight intravenously) or placebo. The primary outcome was mechanical ventilation or death by day 28 and the results showed that although tocilizumab reduced the likelihood of progression to the composite outcome of mechanical ventilation or death, it did not improve survival.
In the REMDACTA study, patients were again randomised (2:1) to tocilizumab plus remdesivir or placebo plus remdesivir. The primary outcome was the time from randomisation to hospital discharge or “ready for discharge” (category 1 on a 7-category ordinal scale of clinical status) to day 28. As with the other trials, when published, the authors concluded that ‘tocilizumab plus remdesivir did not shorten time to hospital discharge or “ready for discharge” to day 28 compared with placebo plus remdesivir.’
However, despite these somewhat negative findings, a systemic review assessing the efficacy of IL-6 antagonists in patients hospitalised for COVID-19, in nearly 11,000 patients, concluded that ‘administration of IL-6 antagonists, compared with usual care or placebo, was associated with lower 28-day all-cause mortality.’
Details of the revised indicated use in COVID-19 can be found in the summary of product characteristics.