The Covid-19 pandemic profoundly impacted global health and fundamentally altered our comprehension of infectious diseases and pandemic control. Amidst the spotlight on vaccine development, therapeutic interventions remained a critical, yet often overlooked, aspect of pandemic preparedness. Here, Gerry Hughes explores the importance of therapeutic technologies for future pandemics and the pathogens and candidate therapies that clinicians should take note of.
The Covid-19 pandemic catalysed a rapid advancement in our understanding of viral infections, yielding valuable insights on managing a modern-age pandemic. While the concept of medical countermeasures encompassing diagnostic, preventative and treatment interventions for public health emergencies is not novel, the pandemic brought it back sharply into focus.
Early in the pandemic, decades of preceding research on messenger Ribonucleic Acid (mRNA) vaccines expedited the development of Covid-19 vaccine technologies, and repurposed drugs like dexamethasone and tocilizumab proved effective in treating hospitalised Covid-19 patients.
However, identifying evidence-based therapeutics posed challenges. Agents such as hydroxychloroquine and lopinavir/ritonavir, initially promising, yielded unsatisfactory results in clinical trials, and remdesivir demonstrated limited efficacy. Newer drugs such as molnupiravir and nirmatrelvir wouldn’t emerge as therapeutic options until later in the pandemic, and they remain prohibitively expensive in many cases.
These shortcomings in Covid-19 therapeutics were discussed at a recent virtual panel discussion entitled ‘Fair Treatment: The Place of Therapeutics in Future Pandemics’. Professor Sharon Lewin, director of the Cumming Global Centre for Pandemic Therapeutics (CGCPT) at the Peter Doherty Institute for Infection and Immunity, summarised that Covid-19 therapeutics ‘came too late, they weren’t fantastic drugs, and they weren’t equitably available across the world’.
Eloise Todd, executive director and co-founder of the Pandemic Action Network, pointed out that loose governance of international efforts to rapidly develop therapeutics during the Covid-19 pandemic left the therapeutics landscape lagging behind the ‘vaccines goldrush’.
Preparation is key
According to the CGCPT, if nirmatrelvir plus ritonavir or molnupiravir ’had been available at scale in July 2020, in line with Covid-19 vaccine approval, it could have prevented millions of deaths globally’.
Recognising the need to better prepare for a future pandemic, the World Health Organization (WHO) is currently leading the charge in developing a global pandemic accord – a framework to help prevent future health emergencies and for countries to adequately respond to any that do emerge.
To achieve this, governmental leaders from 194 countries are currently negotiating the terms of the accord, which involves collating and analysing scientific evidence regarding the potential triggers of future pandemics. Despite ongoing discussions and the intention to finalise the terms at the 77th World Health Assembly held in Geneva earlier this year (27 May – 1 June 2024), a final text remains elusive.
While a new set of international health regulations were agreed at the Assembly, the timeline for the terms of the pandemic accord was extended to the next meeting in 2025.
Therapeutics and pandemic management
In its 2023 technical report on the Covid-19 pandemic in the UK, the Department of Health and Social Care described critical lessons learned. It highlighted that emphasis on collaborative partnerships among the National Health Service, funders, academia, the pharmaceutical industry and the general public played a pivotal role in shaping effective pandemic management. This cross-sector collaboration facilitated several impactful strategies:
- Early identification of candidate agents for prevention and treatment
- Rapid clinical trial setup, including the use of platform trials
- Vaccine and therapeutic procurement and deployment in a globally competitive market.
Looking ahead, the success of Covid-19 vaccination programmes may not be fully replicable in future pandemics. This is because the accelerated vaccine response owed much to decades of prior mRNA research – a unique advantage that may not recur. In such a situation, the availability of safe and effective therapeutics becomes critical.
Professor Michel Kazatchkine, another ‘Fair Treatment’ panellist and a physician and professor of immunology at the Paris Descartes University, France, emphasised the importance of partnering therapeutics with vaccines, not only to improve patient outcomes but to reduce onward infection transmission.
For example, valuable experience was gained from the use of monoclonal antibodies (mAbs) during Covid-19. This experience indicates that mAbs may be best targeted towards diagnostically confirmed disease, increasing disease severity, unvaccinated populations and where the local healthcare system can support delivery of these agents.
Earlier this month, the Independent Panel for Pandemic Preparedness and Response recommended a strategic shift to better align prevention and treatment interventions for deployment in a subsequent pandemic.
The authors noted that during Covid-19, vaccines were ‘one of several tools required to contain a public health emergency, and investment in diagnostics and treatments must also be commensurate’, but the ‘dominant focus on vaccines’ meant ’progress on diagnostics and treatments has been meagre, despite their importance as a first line of response’.
Pathogens to take note of
In a study published earlier this year, 187 infectious disease experts from 57 countries answered a survey to rank various pathogens based on their perceived risk of causing a future pandemic. Results from that study showed that experts were most concerned about influenza, severe-acute-respiratory-syndrome coronavirus (SARS-CoV), SARS-CoV-2 and Ebola.
The Coalition for Epidemic Preparedness Innovations (CEPI) – a global partnership working to accelerate the development of vaccines and other biologic countermeasures against epidemic and pandemic threats – has also raised concerns about several other diseases, namely: Chikungunya, Lassa Fever, Middle East Respiratory Syndrome (commonly known as MERS), Nipah and Rift Valley Fever.
More concerning, perhaps, are novel viral threats with epidemic or pandemic potential that have not yet been characterised – otherwise known as ‘Disease X’. This hypothetical disease exhibits several key features: a viral, zoonotic and mutated pathogen transmitted via respiratory aerosols or droplets.
Candidate therapies for a future pandemic
The International Readiness for Preventing Infectious Viral Disease (INTREPID) Alliance is a non-profit consortium of innovative biopharmaceutical companies dedicated to accelerating the pipeline of antiviral treatments to help protect the world ahead of future pandemics. Part of their mission is to accelerate the collective understanding of antiviral research and development by identifying promising clinical and preclinical compounds.
In April 2024, the INTREPID Alliance published an updated review of antiviral compounds in clinical development for 12 priority virus families of pandemic potential. Of 41 compounds at various phases of clinical development, the INTREPID Alliance has identified 11 of these (see Table 1) as showing promise in fulfilling a ‘100 Days Mission Ready’ brief.
The goal of the 100 Days Mission is to prepare as much as possible so that within the first 100 days of a pandemic threat being identified, key therapeutic interventions can be made available in a safe, effective and affordable way.
3CLPRO is a popular drug target given that its enzymatic activity is critical for the formation of functional viral proteins during viral replication. Almost all the other targets described in Table 1 are enzymes important for functional viruses.
Table 1: 100-Day Mission Ready antiviral compounds in clinical development*
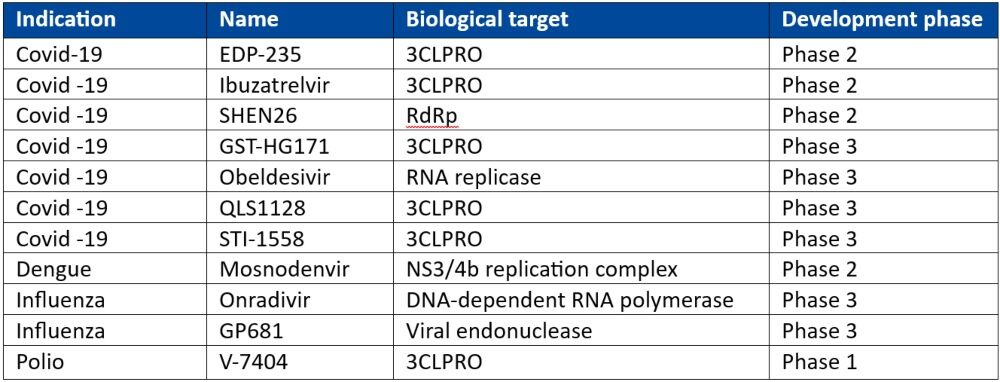
3CLPRO: 3C-like Protease; DNA: deoxyribonucleic acid; RdRp: RNA-dependent RNA polymerase; RNA: ribonucleic acid
*Adapted from the INTREPID alliance Antiviral Clinical Development Landscape – Second Edition
Navigating the way forward for therapeutic interventions
In their closing remarks during the Fair Treatment panel, speakers underscored the intricate challenge of balancing prevention and treatment strategies for the next pandemic. For example, one strategy may involve the use of the aforementioned mAb technology to potentially confer immediate immunity to disease while awaiting a vaccine response. Equally crucial is ensuring equitable access to these interventions.
Strategic investment will play a pivotal role in navigating these challenges, but clinicians and researchers can also play an important part in pandemic preparedness by becoming more engaged with research and development platforms.
Much of the work being undertaken explores emerging approaches – such as gene editing and gene silencing – to directly target pathogen genetic code with the aim of enhancing the innate immune response against entire virus families. Developing cost-effective, broadly active biologics such as antibodies is also a key focus.
What’s more, while it’s not expected as a panacea, the anticipated global pandemic accord agreement within the next 12 months will hopefully provide a robust platform for future-proofing another pandemic, including strategies for improved therapeutic preparedness.