This website is intended for healthcare professionals only.
Take a look at a selection of our recent media coverage:
5th December 2024
Genetics partly influence the shape of the heart’s left and right ventricles, a study shows for the first time, with the findings potentially helping to refine cardiovascular disease (CVD) risk assessment in clinical settings.
Variations in heart shape were more strongly related to cardiovascular risk factors and more predictive of major adverse cardiovascular events than traditional measures of cardiac structure such as mass and volume, researchers wrote in the journal Nature Communications.
‘Genetic associations with heart shape variation may therefore provide information not found from genetic analyses on standard cardiac structural phenotypes and may assist in understanding the mechanisms underlying the development of CVD,’ they said.
The UK and Spanish research team used cardiac magnetic resonance images from 45,683 individuals from the UK Biobank – a large-scale biomedical database and research resource – to create 3D models of hearts before using principal component analysis (PCA) to examine the characteristics of heart shapes.
The analysis identified principal components including heart size, apex-base length and anterior-posterior width.
Genome-wide associated studies were then performed on the first 11 principal components, that captured 83.6% of shape variance, reported the researchers from King’s College London, Queen Mary University of London, and University College London, UK, and University of Zaragoza and University Hospital of A Coruña, Spain.
Through this statistical analysis, they identified 43 significant genomic loci, 14 of which were previously unreported for cardiac traits.
‘This is the first study to examine the genetic architecture of multi-dimensional biventricular heart shape using PCA,’ they wrote.
Most of the principal components were found to be moderately heritable, consistent with previous traditional measures of cardiac structure.
The principal components also demonstrated significant associations with cardiometabolic diseases, including atrial fibrillation and myocardial infarction.
Study co-author Professor Patricia B. Munroe, professor of molecular medicine and centre lead for clinical pharmacology and precision medicine at Queen Mary University of London, said the study provided new information about how researchers considered CVD risk.
‘We’ve long known that size and volume of the heart matter, but by examining shape, we’re uncovering new insights into genetic risks,’ she said.
‘This discovery could provide valuable additional tools for clinicians to predict disease earlier and with more precision.’
Dr Richard Burns, statistical geneticist at Queen Mary University of London, said: ‘This study sets an important foundation for the exploration of genetics in both ventricles. The study confirms that combined cardiac shape is influenced by genetics, and demonstrates the usefulness of cardiac shape analysis in both ventricles for predicting individual risk of cardiometabolic diseases alongside established clinical measures.’
The researchers are keen for further studies to be undertaken on how these findings could be integrated into clinical practice.
And future research could also further examine the anatomical cardiac shape features informed by the identified principal components, they suggested.
Mechanistic studies are also needed to examine the direction of effect between multidimensional heart shape and cardiometabolic disease, the researchers added, including further exploration of the functionality of the identified candidate genes.
The findings follow another study drawing on UK Biobank data published earlier this month which found adults who have irregular sleep patterns were 26% more likely to have a major cardiovascular event than those with a regular sleep-wake cycle.
Published in the Journal of Epidemiology & Community Health, the researchers designed an observational study involving 72,269 people from the UK Biobank aged between 40 and 79 with no history of cardiovascular disease.
For moderately irregular sleepers, meeting sleep duration recommendations could largely offset the elevated risk of cardiovascular events, the researchers found.
7th November 2024
An ‘early warning system’ for future pandemics is to be rolled out in the UK to monitor threats, prevent disease and protect the public, the Department of Health and Social Care (DHSC) has announced.
The surveillance system will be created via the expansion of NHS England’s respiratory metagenomics programme, led by Guys and St Thomas’ NHS Foundation Trust. It uses technology created by life sciences company Oxford Nanopore to analyse genes and pathogens to rapidly diagnose cancers and rare and infectious diseases and match patients with the right treatments within six hours.
The expansion of the programme will also allow potential outbreaks of bacterial or viral diseases to be monitored across the country, alongside antimicrobial resistance.
The technology was initially piloted at St Thomas’ hospital and will be rolled out across 30 NHS sites. Data will be given to the UK Health and Security Agency to allow for quicker detection and action on emerging infectious diseases.
The programme is a partnership between the government, Genomics England, UK Biobank, NHS England and Oxford Nanopore.
Health secretary Wes Streeting said: ‘If we fail to prepare, we should prepare to fail. Our NHS was already on its knees when the pandemic struck, and it was hit harder than any other comparable healthcare system.
‘We cannot let history repeat itself. That’s why this historic partnership with Oxford Nanopore will ensure our world-leading scientists have the latest information on emerging threats at their fingertips.’
Professor Dame Sue Hill, chief scientific officer for England, said: ‘This strategic partnership will build upon our expertise in infectious disease genomics, representing a significant leap forward in our ability to protect public health and save lives.
‘By integrating cutting-edge technology into 30 NHS sites across the country, we are not only enhancing our capacity to rapidly diagnose and treat severe respiratory infections, but also creating a crucial early warning system for new and emerging infectious diseases.’
Professor Susan Hopkins, chief medical advisor at the UK Health Security Agency, said: ‘Enhancing the capacity for the NHS to determine new and emerging pathogens causing severe acute respiratory infections will improve the detection and emergence of infections.
‘As part of the 100 days mission, this will enable the development of effective diagnostics for novel pathogens and enhance our pandemic preparedness.‘
Professor Ian Abbs, chief executive of Guy’s and St Thomas’ NHS Foundation Trust, said: ‘We’ve been working on the respiratory metagenomics programme for over four years and have clearly seen the benefit to our patients. It’s a momentous day now that we can ensure other hospitals, and more patients, can also benefit from faster and more accurate treatment for severe respiratory conditions thanks to new genomic technology.’
A version of this article was originally published by our sister title Healthcare Leader.
30th October 2024
With another successful ESC Congress under its belt, the European Society of Cardiology’s new president Professor Thomas Lüscher speaks to Helen Quinn about the current challenges and opportunities in European cardiology, his highlights from the congress and his thoughts on the future of cardiovascular care.
In September 2024, at the European Society of Cardiology (ESC) annual congress, delegates welcomed Professor Thomas F. Lüscher as their new president and it’s a role he is excited about taking on.
Professor Lüscher is a world-renowned cardiologist, ranking in the top 0.5% globally of most cited scientists and currently a consultant cardiologist and director of research, education and development at the Royal Brompton and Harefield hospitals in London and professor at King’s College London, UK.
Having been involved with the ESC for many years, Professor Lüscher has chaired various working groups, became vice president in 2003 and then editor-in-chief of the European Heart Journal in 2008 – a position he held for 11 years. He describes the society as ‘a fantastic success story’ that has evolved from ‘a small club of friends into the largest and most influential society in medicine’.
With seven associations, seven councils, 15 working groups, 57 national societies, 47 affiliated national societies, 17 journals, 18 textbooks, an annual congress and nine speciality congresses, the ESC works hard to improve cardiovascular care and patient outcomes throughout Europe.
‘[It’s] an institution that dominates the field in a positive manner by providing guidelines, education and registries to improve the burden of cardiovascular disease. So, it’s a really exciting position I have,’ Professor Lüscher says.
Cardiovascular disease is still the leading cause of morbidity and mortality in Europe, and there are significant challenges facing the field. In the past, support from the EU has favoured oncology over cardiovascular healthcare. To try to change this imbalance, the ESC has responded by putting together a cardiovascular health plan, which has been submitted to the EU Council of Health Ministers to raise the profile of research and increase the quality and equality of care patients receive.
‘We hope this will impact the support for cardiovascular science and education in the future,’ Professor Lüscher says. ‘Europe has had a fantastic history. Most of the interventions have been invented in Europe, starting with pacemakers, atrial fibrillation ablation, percutaneous coronary intervention, transcatheter aortic valve implantation and MitraClip. It’s quite an amazing story.’
Today, however, innovation and development are hampered by regulations, according to Professor Lüscher. At the same time, the Food and Drug Administration in the US has become more lenient and much quicker and effective in approving drugs and trials.
‘I’m concerned that the speed and impressive innovation we have delivered over the last 200 years may be fading a little bit. There has been a bit of a shift from Europe to the US. [There are] a lot of rules and regulations in the EU and the UK,’ explains Professor Lüscher.
A lack of centralised device regulation in Europe is also impeding developments in field. Consequently, the ESC is working constructively with the European Medicines Agency and the Notified Bodies to make Europe fitter for innovation.
For some patients, differences in access to care is one of the main barriers to improving cardiovascular health across the continent. Such inequalities are highlighted in the ESC’s publication ‘Atlas of Cardiology’, which gives a picture of the current state of cardiovascular across Europe and shows vast differences in modern management options for cardiovascular conditions in different countries.
Patients in countries like Germany, Switzerland, Scandinavia and the Netherlands have good access to the latest treatments and medications. In other European countries, access is much more difficult, with many patients – particularly those in Eastern Europe – missing out.
And in the UK, for example, there is a concern that lower social classes have limited access to the latest cardiovascular treatments, Professor Lüscher explains, with deprived areas experiencing worse levels of care and, in turn, worse outcomes.
‘If you have severe heart failure, you might need a left ventricular assist device and in many countries that’s not available. Also, some novel, more expensive drugs are not available in certain countries,’ Professor Lüscher says. ‘There’s a huge heterogeneity in access to treatment across European countries. These are ethical concerns for medicine that, by nature, is a humanistic profession. The ESC tried to address this problem.’
The European Union has tried to overcome these inequalities in care by putting pressure on the prices of medications. There is also pressure on patent durations to make generic therapies available more easily and earlier, which is beneficial in the short term, but it is something that Professor Lüscher worries will obstruct innovations in the long term.
‘In the end, this is an economic problem,’ Professor Lüscher says. ‘There’s a close correlation between gross national product and availability of medical services, and currently in Europe the economy is not doing well. In many countries, we have issues with the economy that reflect on the service for patients.’
There is, however, much to be excited about in the field of cardiology, with many innovations and new research shared at the ESC Congress 2024. For Professor Lüscher, two significant potential developments excited him the most.
The first is the development of genetic tools as therapeutic agents to treat and prevent cardiovascular disease. This cutting-edge approach focuses on the use of antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs), which can block the production of certain proteins in the body and currently mainly target the liver.
‘The liver has specific receptors, in particular the asialoglycoprotein receptor, mainly expressed in hepatocytes. So, once linked with a GalNac residue, you can direct these double-stranded RNAs specifically to the hepatic cells. Then they bind to the RISC complex within the cell and inhibit the translation of a transcript to a protein over several months,’ Professor Lüscher explains.
This process enables a long-lasting therapeutic approach. There are now siRNAs for PCSK9, which lower low-density lipoprotein (LDL) plasma levels for six months, and others, including a new development for lipoprotein(a). In addition, siRNA therapies can target angiotensinogen to lower blood pressure for several months. Other siRNAs, like those that reduce transthyretin (TTR), help treat ATTR amyloidosis by preventing the formation of harmful amyloid deposits.
Gene editing tools, such as CRISPR-Cas9, are also emerging, which can precisely modify nucleoid acid sequences in the DNA. In animal trials, this tool has been used to permanently block the production of PCSK9, preventing it from binding to LDL receptors and thus lowering cholesterol levels and potentially offering a one-off, lifelong treatment.
‘The long-term vision is that we cure rather than treat. These genetic tools are a completely new chapter in pharmacotherapy,’ Professor Lüscher says.
A second area of innovation that will continue to be incredibly influential in cardiovascular medicine is the development of artificial intelligence (AI) and machine learning. As part of his presidency, Professor Lüscher has set out his vision for the digital transformation of cardiology in Europe.
Beginning with online consultations, he believes AI has much to offer clinicians and patients. ‘With an algorithm, you can analyse the face of a person, see the pulse, see the wrinkles, see the amount of sweat, and you can make outcome predictions,’ he says.
‘AI analyses any sort of picture, not just faces, but echocardiograms, CT scans, MRIs, nuclear scans, pathology specimens, biopsies – anything that’s visual and can also diagnose patients,’ he adds.
Analysing the human voice is also possible using AI, which can be incredibly helpful for cardiovascular diagnosis by identifying atrial fibrillation and arrhythmias through variations heard in the vocal cords as well as congestion caused by heart failure.
Professor Lüscher believes AI algorithms will become important ‘co-pilots’ for clinicians, prompting them to think about diagnoses they may have missed. Other algorithms can read reports shared as part of a referral, giving summaries and analysing volumes from images in seconds that would otherwise take clinicians significant chunks of time.
‘It makes us faster and more precise, provided the algorithms are good,’ Professor Lüscher says. ‘Algorithms that are not good or false can potentially kill patients. These algorithms have to travel well and work in different geographical areas and countries, otherwise it’s not acceptable.’
As such, the ESC is involved in developing quality standards for algorithms across Europe and only uses algorithms that they can show are well-verified in independent cohorts.
Amidst the innovations and new pathways, there will inevitably be challenges ahead, but there is much to look forward to in cardiology as Professor Lüscher begins his two-year presidential journey.
4th March 2024
While a significant proportion of patients with myocardial infarction are prescribed clopidogrel, common genetic variants mean that some people – and more from certain ancestry groups – may not see its preventative benefit.
Here, Dr Emma Magavern, clinical research fellow for the Centre of Clinical Pharmacology and Precision Medicine at William Harvey Research Institute, Queen Mary University of London, UK, discusses her most recent findings and the value of clinicians adopting pre-emptive pharmacogenomic testing.
As clinicians, we know that there is inter-individual variability in medication response. Different people respond differently to the same medication; some take medication and do not experience the intended benefit, and some people unfortunately experience adverse drug reactions (ADRs). Some of this variability in response is due to genetics.
We already use other information known to impact on risk of ADRs from a medication in our clinical practice. Examples include checking liver and renal function before prescribing medicines.
By using information on very common genetic markers to inform prescribing, we can optimise the benefits of medications and minimise the risk of side effects. This area, known as pharmacogenomics, has however been slow to enter mainstream clinical practice.
The recently published PREPARE trial showed that a pre-emptive pharmacogenomic testing approach, where the genotype informs prescribing, can decrease ADRs by one-third in a multi-centre prospective clinical trial setting.1
ADRs pose significant problems for patients and take a large toll on healthcare systems. Studies estimate ADRs are responsible for 6.5% of hospital admissions in the UK and cost more than £2bn a year to the NHS.2,3 So there is clearly much room for improvement.
People from certain ancestry groups may have a higher risk of experiencing a side effect or not benefiting from medication due to common genetic variants. An example is illustrated by the use of clopidogrel after a myocardial infarction.
Clopidogrel is a commonly prescribed P2Y12 inhibitor used for secondary prevention after an initial ischaemic event.4 It is a prodrug and must therefore be activated in the body, via metabolism by cytochrome P450 2C19 (CYP2C19) in the liver, to achieve potency. Studies have shown that 60–70% of people who suffer from a myocardial infarction are prescribed clopidogrel.5
The CYP2C19 enzyme is encoded by the CYP2C19 gene, which is highly polymorphic.6 People with CYP2C19 loss of function (LOF) variants have higher on-treatment platelet reactivity with clopidogrel and are at a greater risk of secondary ischaemic events.7
LOF variants in CYP2C19 are common – present in approximately one in three people of European ancestry.7 Therefore, genetic variants resulting in individuals not being able to activate clopidogrel are common.
However, these variants are even more common in certain ancestral groups. Genetic changes that lead to poor activation of clopidogrel are most common in Asian and Oceanic ancestry groups.8
So, these ancestry patients have a higher risk of clopidogrel being ineffective than European ancestry participants, in whom antiplatelets were predominantly trialled, and therefore might benefit most from the use of genetics to help determine clopidogrel safety and efficacy (see Table 1 below).
Few studies have linked genetic data from under-represented populations with medication exposure and health outcome data. The only study with a substantial Asian population was focused on stroke rather than myocardial ischaemia and undertaken in east Asia.
Table 1. Studies supporting clopidogrel licensure as listed in the European Medicines Agency summary of product characteristics9–16
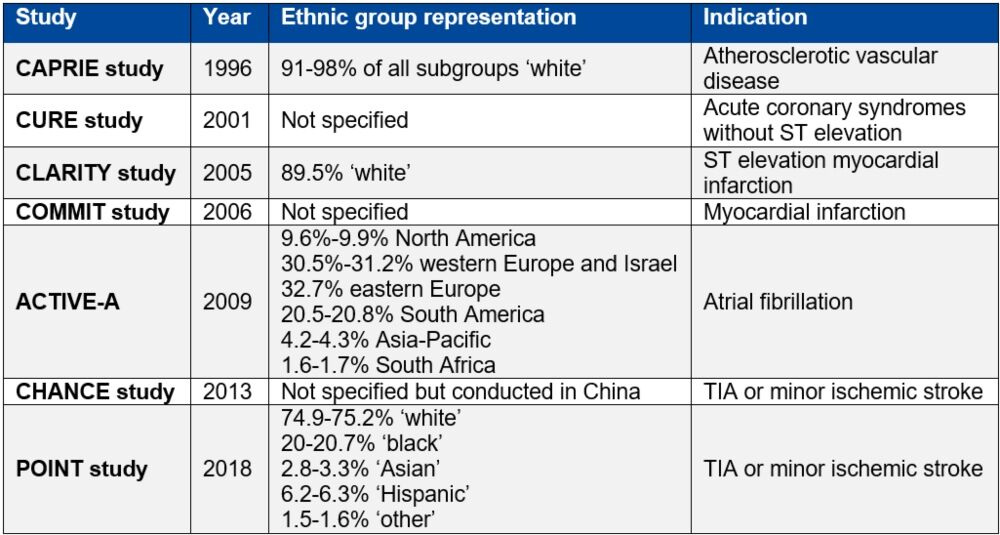
Table reproduced from Magavern E et al. JACC Adv 2023;2(7):100573. Copyright © 2023, The Authors. Open Access Article under the CC BY License.
The British-Bangladeshi and British-Pakistani populations in the UK are known to experience high rates of cardiovascular disease. The Genes & Health study recruited more than 50,000 people from this population and linked genotype data with national electronic health records data and primary care prescription records.17
In an analysis of 44,396 participants, we found that 57% of people in this group had a CYP2C19 LOF variant.18 Of these, 13% had two copies of an LOF variant – one from each parent (Figure 1).
Figure 1. Prevalence of CYP2C19 LOF alleles in the Genes & Health study’s South-Asian ancestry population
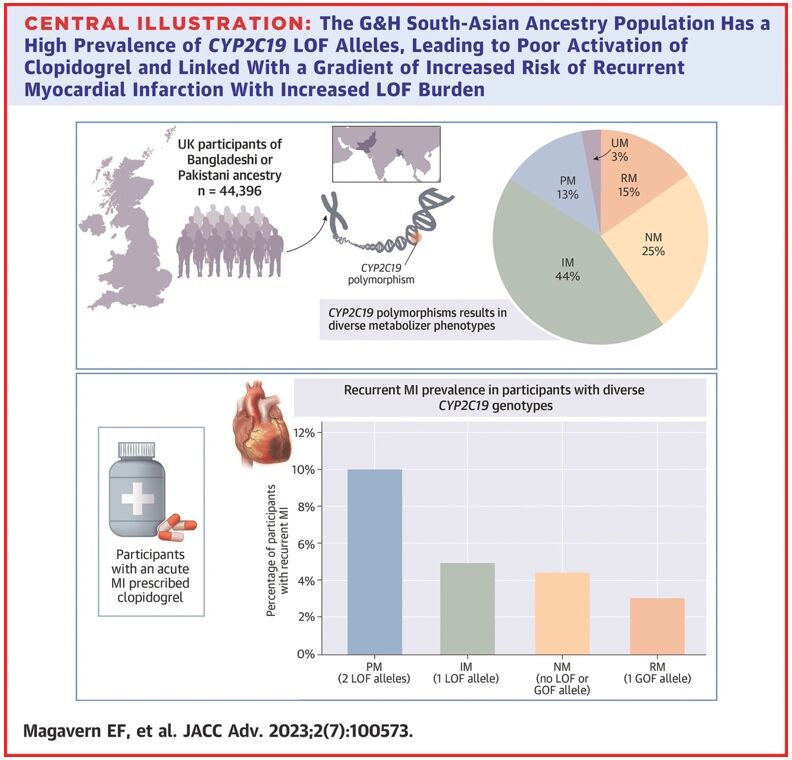
Reproduced from Magavern E et al. JACC Adv 2023;2(7):100573. Copyright © 2023, The Authors. Open Access Article under the CC BY License.
We identified people in this group who had experienced a myocardial infarction and were prescribed clopidogrel by their GP (697 participants). Those with a recurrent myocardial infarction were almost four-times more likely to have two LOF CYP2C19 alleles as compared with none. A total of 69% of participants who had a myocardial infarction were prescribed clopidogrel.18
This study showed how common genetic resistance to clopidogrel is in this population. It also demonstrated the widespread use of clopidogrel and linked genetic resistance with a higher risk of recurrent myocardial infarction in this population for the first time, using real-world data.
While a precision prescribing approach using CYP2C19 testing would benefit patients from all ancestries, it would disproportionately benefit the South-Asian ancestry population due to the higher prevalence of both coronary artery disease and genetic resistance to clopidogrel in this population.
Genetic testing may soon be available within the NHS to determine if stroke patients would benefit from clopidogrel.19 This means that some patients might already know their CYP2C19 genotype when they present with a secondary ischaemic event.
This information could subsequently be used to administer the most effective medicine and reduce their risk of further ischaemic events.
For pharmacogenetic testing to benefit patients who have had a myocardial infarction, several things must occur:
There should also be discussion with the public around this use of genetic testing, to raise awareness and design clinical pathways fit for purpose. These dialogues must include historically under-represented ancestry groups and build trust.
The goal is to make genetic testing possible in routine clinical care to help optimise antiplatelet choice after an ischaemic event and to ensure better outcomes for all, with a particular focus on improving health equality for the British South-Asian ancestry population.
Emma Magavern MD MSc MRCP
Clinical Research Fellow, Centre of Clinical Pharmacology and Precision Medicine, William Harvey Research Institute, Queen Mary University of London, UK
26th May 2023
A genetic ‘biobank’ will be launched on 1 June 2023 by the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) to understand how a patient’s genetic make up can impact the safety of their medicines.
Known as the Yellow Card biobank, the biobank will contain genetic data and patient samples and be used by scientists to determine whether a side effect from a medicine was caused by a specific genetic trait.
A joint venture with Genomics England, the genetic research resource is the first of its kind to be launched by a drug safety regulator.
Operating alongside the MHRA’s existing Yellow Card reporting site for suspected side effects and adverse incidents involving medicines and medical devices, the new venture forms part of a long-term vision for more personalised medicine approaches.
By understanding the underlying mechanism of an adverse drug reaction (ADR), it is hoped that pharmacogenetic testing strategies could be developed so that ADRs could be prevented rather than requiring a reactive approach.
In turn, this will enable doctors to target prescriptions so UK patients will receive the safest medication for them, based on their genetic makeup.
‘We are excited by the upcoming launch of the Yellow Card biobank, which demonstrates that we are at the absolute forefront of innovation in the field of drug safety monitoring,’ said Dr June Raine, MHRA Chief Executive. ‘Almost a third of adverse reactions to medicines could be prevented with the introduction of genetic testing… This has the potential to transform our safety monitoring activities – enabling us to meet a real need by using high-quality patient data to reduce side effects of medicines.’
Recruitment for the biobank will commence on 1 September and participants will be visited at home by a nurse who will take a blood sample to be added to the biobank. The sequencing of participants’ genetic material will begin in spring 2024. Initial research findings are due to be published in 2025.
The pilot phase will start by looking at allopurinol and related rare, severe skin reactions including Stevens-Johnson syndrome and toxic epidermal necrolysis. Other topics of focus for the pilot phase will be confirmed in due course.
Genomics England will support the MHRA with the sequencing and storage of genetic material through use of its well-established and secure infrastructure.
Commenting on the ‘transformative partnership’ with the MHRA, Professor Matt Brown, Chief Scientific Officer for Genomics England, added: ‘Many [severe ADRs] are influenced by underlying genetic risk factors, substantially heightening an individual’s vulnerability.
‘By joining forces with the MHRA, we are poised to gain greater understanding of these genetic influences – discoveries that will be vital if we are to move to harness the power of genomics to proactively protect patients from these harms.
‘Together, we hope that this is the first step towards redefining the future of drug safety.’
ADRs continue to be a significant burden on the NHS and account for one in 16 hospital admissions.