This website is intended for healthcare professionals only.
Take a look at a selection of our recent media coverage:
31st August 2023
Mitazalimab has received orphan drug designation status by the European Medicines Agency (EMA) for the treatment of patients with pancreatic cancer, its manufacturer Alligator Bioscience has announced.
A human CD40 agonistic antibody targeting CD40, mitazalimab kickstarts the cancer-immunity cycle by priming and activating tumour-specific T cells. Targeting CD40 with mitazalimab has the potential to augment responses to chemotherapy.
Pancreatic ductal adenocarcinoma (PDAC) is known to be the fourth-leading cause of cancer related mortality in the world and has a poor prognosis, with a five-year survival rate of below 5%.
To qualify for the EMA’s orphan designation, a medicine must be intended for the treatment, prevention or diagnosis of rare, life-threatening or chronically debilitating diseases that affect fewer than five in 10,000 persons in the EU. Medicines that meet these criteria are eligible for financial and regulatory incentives that include 10 years of marketing exclusivity in the EU after product approval.
The EMA orphan drug designation follows a similar approval by the FDA in May 2023.
Commenting on the EMA approval for orphan drug designation status, Søren Bregenholt, CEO of Alligator Bioscience, said: ‘We are very pleased that the European Medicines Agency has granted orphan designation to our lead asset mitazalimab in the treatment of pancreatic cancer.
‘It is our second orphan designation this year following the FDA‘s decision to grant us [orphan drug designation] in May, meaning mitazalimab now has stronger commercial protection through market exclusivity in these two key markets. This latest designation adds to the momentum we are building in our efforts to bring this promising drug candidate to market.‘
Mitazalimab is currently being evaluated in the phase 1b/2 OPTIMIZE-1 trial in combination with mFOLFIRINOX chemotherapy for adult patients with previously untreated metastatic PDAC.
In the trial, participants receive mitazalimab and mFOLFIRINOX via intravenous infusions following a 14-day cycle schedule. Mitazalimab is administered two days after mFOLFIRINOX, except for the first cycle of 21 days, where the drug is administered on days one and 10 with infusion of mFOLFIRINOX starting on day eight.
Interim results from OPTIMIZE-1 released in June 2023, showed a deepening of tumour response and an increase in the objective response rate (ORR) from 52% to 57% in a cohort of 23 patients.
In the full study cohort of 57 patients, there was an interim ORR of 44%, and this is expected to further improve with longer follow-up. A median duration of response of 8.7 months was also reported.
21st September 2022
A deep learning-based tool has been shown to accurately detect pancreatic cancers that are less than 2 cm and which can often be missed during an abdominal CT scan according to the findings of a retrospective study by Taiwanese researchers.
Pancreatic cancer has a poor prognosis and is the 12th most common cancer worldwide and in 2020 there were more than 495,000 new cases and an estimated 466003 global deaths. However, 5-year survival is poor and data for the UK suggests that only 7.3%)of people diagnosed with the cancer in England survive for five years or more.
The clinical diagnosis of pancreatic cancer is challenging as patients often present with non-specific symptoms with nearly a third of patients clinically misdiagnosed. Imaging has a crucial role to play in diagnosis though one retrospective analysis of different imaging modalities revealed that 62% of cases were missed and 46% misinterpreted, with 42% of cases missed because the tumour was less than 2 cm.
Previous research using a deep learning-based convolutional neural network, showed that the technology could accurately distinguish pancreatic cancer on computed tomography (CT) with acceptable generalisability to images of patients from various races and ethnicities.
However, in that study, segmentation of the pancreas, i.e. identifying that the region on a CT scan which actually is the pancreas, was performed manually by radiologists. But would it be possible for a deep learning-based tool to enable segmentation and to detect the presence of pancreatic cancer?
This was the question addressed in the current study by the Taiwanese team. They used contrast-enhanced CT collected from patients who had been diagnosed with pancreatic cancer and compared these with CT scans of non-cancer, control patients.
The deep learning-based tool was initially trained and validated on samples with and without cancer and then tested in a real-world set of CT scans and its performance assessed based sensitivity, specificity and accuracy.
Deep learning tool and prediction of small pancreatic tumours
A total of 546 patients with a mean age of 65 years (46% female) who had pancreatic cancer with a mean tumour size of 2.9 cm and 733 control patients were used in the training, validation and test set.
In a nationwide test set that included 669 cancer patients and 804 controls, the deep learning-based tool distinguished between CT malignant and control samples with a sensitivity of 89.7% (95% CI 87.1 – 91.9) and a specificity of 92.8% (95% CI 90.8 – 94.5) and an accuracy of 91.4%.
When comparing the tool with radiologists, the corresponding sensitivities for the local test set (109 cancer and 147 control patients) were 90.2% and 96.1% for the tool and radiologists respectively and this difference was not significant (p = 0.11).
The tool had a sensitivity of 87.5% (95% CI 67.6 – 97.3) for a malignancy which was smaller than 2 cm in the local test set although this was slightly lower (74.7%) in the nationwide test set.
The authors concluded that their tool may be of value as a supplement for radiologists to enhance detection of pancreatic cancer although further work was needed to examine the generalisability of the findings of other populations.
Citation
Chen PT et al. Pancreatic Cancer Detection on CT Scans with Deep Learning: A Nationwide Population-based Study Radiology 2022
17th March 2022
The use of a faecal microbiota signature can be used for the screening and early detection of patients with pancreatic adenocarcinoma. This was the finding of a study by researchers from the Structural and Computational Biology Unit, Baden, Germany.
Pancreatic ductal adenocarcinoma accounts for more than 90% of pancreatic cancer cases and is a highly aggressive and lethal cancer due to both the lack of early detection and limited response to treatments.
Furthermore, the majority of patients with pancreatic cancer are asymptomatic until the disease reaches an advanced stage and there is no standard programme for screening high-risk patients. Currently, the only biomarker approved for pancreatic ductal adenocarcinoma is serum carbohydrate antigen (CA19-9) although its use is limited by low sensitivity and specificity.
Some evidence suggests that there is a relationship between the duodenal microbiota in pancreatic head cancer patients that could be useful in future trials investigating the role of faecal microbiota in pancreatic cancer. Nevertheless, translation of the potential changes in gut microbiota and its relationship to pancreatic cancer has been largely unexplored.
For the present study, the German researchers took faecal samples from normal and cancerous pancreatic tissue and assessed the microbial composition using whole-genomic sequencing.
They recruited three groups of patients: newly diagnosed adults with pancreatic cancer but before they had started treatment; individuals for which pancreatic cancer was suspected and finally a cohort with chronic pancreatitis.
The results from these patients were used in the discovery phase of the study whereas a second patient group was used for validation purposes. The sensitivity and specificity were determined using the area under the receiver operating characteristic curve.
Faecal microbiota and pancreatic ductal adenocarcinoma
A total of 57 newly diagnosed treatment naive and 29 with confirmed pancreatic cancer were included in the analysis.
The faecal microbiota composition was found to be significantly different in patients with pancreatic adenocarcinoma compared to both controls (p < 0.0001) and from patients with chronic pancreatitis.
A faecal metagenomic classifier identified a pancreatic ductal adenoma with an area under the curve (AUC) of 0.84 based on the presence of 27 bacterial species. However, with addition of CA19-9 levels, this increased the AUC to 0.94. Using a separate sample of patients to validate the classifier, it was found that the AUC was 0.83.
In a discussion of their findings, the authors suggested that the metagenomic classifier was able to robustly and accurately predict pancreatic ductal adenocarcinoma based on the composition of faecal microbiota species and that addition of CA19-9 data (which is already an approved biomarker) further enhanced the accuracy of the model.
They concluded that the microbial panel they had identified could provide future entry points for disease prevention and therapeutic interventions.
Citation
Kartal E et al. A faecal microbiota signature with high specificity for pancreatic cancer Gut 2022
14th January 2022
A radiomics nomogram which incorporates the computed tomography (CT) derived radiomics signature and CT-reported lymph node (LN) status, provides favourable preoperative predictive accuracy of LN metastases in patients with pancreatic ductal adenocarcinoma (PDAC). This was the finding from a retrospective study by a team from the Department of Radiology, Changhai Hospital, Shanghai, China.
Pancreatic ductal adenocarcinoma (PDAC) is the fourth-leading cause of cancer related death in the world with a 5-year survival rate of less than 5%. Moreover, pancreatic cancer is a highly lethal disease, for which mortality closely parallels incidence such that every year, more than 350,000 people worldwide are diagnosed and more than 340,000 die of the disease.
The presence of lymph node metastases have been found to be present in up to nearly 68% of patients leading to a significantly poorer prognosis.
The use of multi-slice computed tomography (MSCT) is seen as the best initial diagnostic test for pancreatic cancer although according to a 2014 systematic review, the technique has a low diagnostic accuracy for the detection of LN metastases.
One alternative strategy is the use of radiomics, which represents a quantitative and non-invasive approach to imaging and aims to enhance the existing data to clinicians by means of advanced mathematical analysis. While the use of radiomics has been found to be of value in detection of some cancers, it has not been used for predicting LN metastases in those with PDAC.
For the present study, the Chinese team, aimed to develop and validate a radiomics nomogram that incorporated a radiomics signature and CT-reported LN status for the pre-operative prediction of LN metastasis in those with PDAC.
Patients who had undergone MSCT were divided into a training and validation cohort with both groups including LN negative and positive individuals. The authors used multivariable logistic regression analysis to develop a model to predict LN metastases and the area under curve (AUC) values used to estimate the model’s sensitivity, specificity and accuracy.
Findings
A total of 225 patients aged between 59 and 64 years of age were split into a training (180) and validation cohort (45). Using univariate analysis, only the radiomics score (rad-score) (p < 0.0001) and CT-reported LN status (p = 0.014) were significantly associated with an increased risk of LN metastases.
In the validation cohort, the radiomics model yielded an AUC of 0.81, giving a sensitivity of 84.2%, a specificity of 69.2% and an accuracy of 75.6%. Using just the radiomics score and CT-reported LN status, decision curve analysis showed that with the nomogram, if the threshold probability was between 0.25 and 0.75, using the nomogram to predict LN metastases added more benefit that a treat-all patients strategy.
They concluded that the radiomics nomogram showed favourable accuracy for the pre-operative prediction of LN metastases in PDAC patients.
Citation
Bian Y et al. Radiomics nomogram for the preoperative prediction of lymph node metastasis in pancreatic ductal adenocarcinoma. Cancer Imaging 2022.
21st November 2019
Professor Matthias Löhr explains why increased awareness of pancreatic cancer and increased investment into the field should become an urgent priority across Europe.
On 21 November 2019, United European Gastroenterology (UEG) and Pancreatic Cancer Europe (PCE) recognises World Pancreatic Cancer Day, a global initiative developed to raise awareness and prompt action against one of the world’s deadliest cancers.
Over the past 50 years, diagnosis and treatment strategies for cancer patients have evolved rapidly, transforming patient outcomes. Despite the major advancements witnessed in other areas of oncology, improvements in pancreatic cancer patient outcomes have largely stood still. In sharp contrast to the remarkable growth in survival rates observed in other disease areas such as lung, breast or prostate cancer, the overall five-year survival rate for patients diagnosed with pancreatic cancer is just 5% across the globe, a figure that has not significantly improved since the 1970s.1
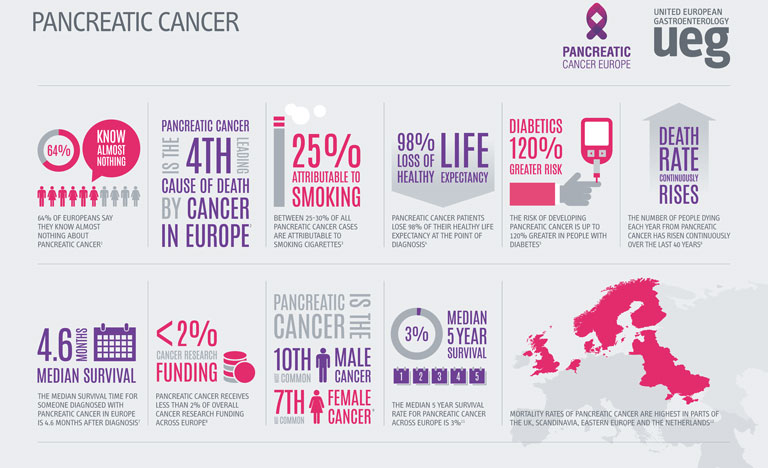
Compounding the threat of these concerning statistics, the incidence and mortality rates related to pancreatic cancer are on the rise globally. A recent study presented at UEG Week Barcelona 2019 revealed that as well as an increase in pancreatic cancer cases, the number of deaths attributable to the disease has risen from 196,000 in 1990 to 448,000 in 2017.1
Whilst a proportion of this increase can be explained by a rising population and increased life-expectancy, age-standardised incidence and death rates for pancreatic cancer had still risen by 12% and 10% respectively over the course of the study.1 Particularly of note, the highest incidence and death rates were recorded in higher-income countries.
Whilst the precise aetiology of pancreatic cancer remains unknown, a number of factors have been linked to the development of the disease. Obesity, an epidemic that has a close association with high-income countries, has been shown to increase a patient’s risk of pancreatic cancer by almost 47%.2 The ever-increasing prevalence of obesity and diabetes across the globe, coupled with an ageing population is set to add weight to the already-heavy burden of pancreatic cancer.
Recent forecasts have predicted that both the number of cases and deaths will increase by 40% by 2035 if preventative measures are not taken.3 With an estimated two-thirds of the risk factors being categorised as potentially modifiable, there is a huge opportunity for both clinicians and the public to promote and partake in lifestyle changes that can significantly reduce the risk of the disease.4
However, a number of pressing challenges still face clinicians and the general public in the identification and treatment of pancreatic cancer. In the earliest stages of the disease, symptoms are often silent or general, making pancreatic cancer a notoriously difficult disease to diagnose.
Perpetuating this problem further, poor public awareness of pancreatic cancer and the absence of a standard diagnostic tool frequently causes delays in identification, allowing the cancer to remain present in the body for many years prior to detection. As a result, only 10-20% of cases are identified in time for curative surgery.5 For those who are not diagnosed in time for resection, violent tumours can persist, which display extreme resistance to treatment, partially explaining the incredibly low survival rates associated with pancreatic cancer.
Despite these daunting statistics, recent progress has provided renewed hope for the future status of pancreatic cancer. Building upon a wealth of established research, a number of incremental improvements have been seen across the field, including the increased efficacy of a range of treatment options.
Traditionally, surgery, chemotherapy and radiation therapy have been the most commonly used tools in the fight against pancreatic cancer. However, the advent of immunotherapy could signify a new frontier for pancreatic cancer treatment.
Recent studies have suggested that combination treatment strategies in conjunction with immunotherapy could potentially yield positive results, improving pancreatic cancer prognosis.6 In order to provide robust and conclusive evidence of the benefits of immunotherapy, a closer examination of this treatment appears to be a worthwhile future endeavour.
In light of the clear need for further research into pancreatic cancer, the lack of funding provided to this area is particularly unsettling. European funding for the disease lies far behind many other cancers with similar mortality rates, receiving less than 2% of all cancer research funding in Europe.7 An increased allocation of EU funds to pancreatic cancer research will allow for the exploration of a number of different treatment options that could significantly improve patient outcomes across Europe.
The Cancer Moonshot programme, a project launched across the US with the aim of reducing mortality rates in several major cancers, represents a promising new development in pancreatic cancer research. The resultant Precision PromiseSM, an adaptive randomised clinical trial platform, allows researchers to evaluate multiple novel therapies to develop effective and ground breaking treatment options for pancreatic cancer.8 The implementation of similar projects across Europe and the wider world should be seen as an essential step in improving pancreatic cancer outcomes across Europe.
Solving the persistently difficult question of how to improve pancreatic cancer care will ultimately require not only the allocation of funds to the field, but a coordinated approach from all areas of society, including the public, academia and industry. Improving awareness of the disease amongst the public, encouraging researchers to enter the field and apply for grants, and incentivising drug companies to focus on drug development, are all crucial steps we need to take if we are to change the course of this destructive and often overlooked disease.
Matthias Löhr MD PhD
Professor of gastroenterology and hepatology, Karolinska Institutet, Sweden, and member of the UEG Public Affairs Committee