This website is intended for healthcare professionals only.
Take a look at a selection of our recent media coverage:
31st January 2025
Speaking at Hospital Healthcare Europe’s Clinical Excellence in Cardiovascular Care event, Dr Rebecca Dobson discussed the acute need to assess cardiovascular risk in cancer care, understanding cardiotoxicity and the importance of multidisciplinary team coordination in cardio-oncology.
Following on from her previous Clinical Excellence session in which she discussed the need and demand for cardio-oncology services, Dr Rebecca Dobson, consultant cardiologist specialising in imaging and cardio-oncology at Liverpool Heart and Chest Hospital, turned her attention to considerations such as baseline assessment, cardiovascular risk factors and the impact of systemic anti-cancer therapy on the cardiovascular system – even years down the line.
Dr Dobson also championed collaborative care between cardiology, oncology, radiology and other fields to ensure patients can continue their cancer therapy alongside cardio-protective therapies to optimise their short- and long-term outcomes.
Trends change, and cancer is becoming more common, but happily cancer survival has also doubled in the UK in the last 40-50 years. It’s really important that we, as cardiologists and cardiology teams, don’t shy away from this group of patients, because there’s a lot we can do from a cardiovascular perspective to help them live long, healthy lives after they’ve had cancer.
The authors of a recent paper in the European Heart Journal looked at all patients with cancer and stratified them according to cancer mortality rate and cardiovascular disease mortality rate.
In one group, the patients sadly have very high cancer mortality rates (70-90%) and, as a cardiologist, there’s very little I can do with that cohort of patients to change the natural trajectory of that disease. The impact from a cardio-oncologist perspective is relatively low.
The second group of patients have a slightly lower cancer mortality rate (40-60%) and an increasing cardiovascular disease mortality rate (10-20%), and there’s more we can do in terms of trying to reduce long-term effects of systemic anti-cancer therapies and reducing the risk of cardiotoxicity to avoid cardiovascular complications.
In the third group, these patients are as likely to die from cardiovascular disease as they are from their cancer – both at 20-30%. It’s really important that we don’t cure these patients’ cancer and then leave them alone. We really need to optimise the cardiac care of these patients to consider them as a whole, not just as a cancer patient.
As a consequence of increasing cancer rates and survival, more patients are being exposed to potentially cardiotoxic therapies, and more people with pre-existing cardiovascular disease – who 30 years ago would have died of their cancer – are now surviving. That’s where the subspecialty of cardio-oncology has come from.
We specifically see three different groups of patients. Firstly, we see patients at the beginning of their cancer journey to risk-stratify them. That could be a patient with any cardiovascular disease who then receives a cancer diagnosis and needs to be risk-stratified and optimised from a cardiovascular perspective. Importantly, there needs to be a discussion with the oncology team about that patient’s cancer therapy to ensure it won’t destabilise their existing cardiovascular disease.
As patients go through their cancer journey, we screen and monitor them to detect cardiovascular injury at earliest possible stage so we can get them on appropriate cardio-protective therapies and reduce the interruptions to systemic anti-cancer therapy.
This has been a big change over the last 10 or 20 years. Historically, if you had cancer and then you developed potential cardiotoxicity, it was a binary decision. Sadly, for these patients, cancer therapies were generally discontinued, whereas now we try to continue cancer therapy wherever possible.
The last group of patients we see within the cardio-oncology service is screening and monitoring patients who are cured of their cancer but are at risk of significant long-term cardiovascular late effects.
Classically, this would be patients who had a haematological malignancy or a bone cancer as children or young adults and received significant amounts of anthracycline chemotherapy. We know that puts them at risk of late-effects even 30 years later.
We don’t want these patients to be discharged and forgotten about but then present in adulthood in heart failure. So, we’ve set up a service, certainly in Liverpool, whereby every patient who has had a significant dose of anthracyclines gets put into a late effects clinic. We see them every three to five years, so they don’t fall off that cliff.
Baseline assessment is a little bit contentious from an oncology point of view, and oncology teams have a lot to do with their patients when they first get their cancer diagnosis. To then ask them to also risk-stratify patients from cardiovascular perspective is challenging.
As a cardio-oncologist, I see my role as facilitating this baseline assessment and I work with the region’s oncology teams to do this.
It helps us consider what changes may be required to systemic anti-cancer therapy and enables us to potentially detect undiagnosed cardiovascular problems at baseline.
We are performing increasingly more investigations on patients receiving chemotherapy and radiotherapy. It can be difficult to interpret these investigations without a baseline for comparison otherwise we won’t know if cancer therapy has caused an issue or whether there was pre-existing disease. It also enables us to reduce the risk of cardiotoxicity as patients move through treatment.
I recommend that you download the European Society of Cardiology’s guidelines on cardio-oncology because it gives a helpful overview. It’s a large document but there’s lots of useful information in there.
When I talk to oncologists, they’ll often ask, ‘how do we assess cardiovascular risk, what is it we need to do?’ The first thing to say is that not every patient needs every test. I’m a real believer in that there’s no point doing a test unless you know what you’ll do with an abnormal result. That’s particularly challenging for oncology teams who aren’t used to dealing with echoes, troponins, NT-proBNPs, cardiac MRI scans or sometimes even ECGs.
It’s really important to tailor the approach to the patient depending on their comorbidities and what systemic anti-cancer therapy is proposed. Things like age, sex, demographics, past medical history, lifestyle factors like smoking or being overweight, a family history – if alarm bells are ringing as the history is being taken and the examination is happening, then you might think that the patient needs to go on to have further complementary tests.
It’s really important when we’re setting up these services that cardiology teams work collaboratively with oncology teams to make sure pathways are in place to help us work out what we’re going to do with the abnormalities.
There’s an easy way of quantifying risk now. If you download the free ESC app, you can access risk calculators. It’s easy to fill out the yes or no questions and it gives you a risk at the end: low, medium, high and very high.
The four outcomes are very arbitrary, but the ESC cardio-oncology guidelines stipulate that this is the approximate risk we should be discussing with patients – high-risk conversations will be very different to low-risk ones.
If you’re low risk, you’ve got a less than 2% chance of developing cardiotoxicity, although note that it’s not zero – I always tell patients we can never say they won’t develop a cardiovascular problem. Low-risk patients should have standard monitoring, and, hopefully, as cardiologists or cardio-oncologists, we don’t need to get involved with them.
Medium-risk patients should have closer monitoring, which will differ depending on your region’s resources. We don’t see medium-risk patients here, but we often speak with oncology teams and suggest closer echo or blood pressure monitoring, whatever the issue might be.
High-risk and very high-risk patients have a substantially increased risk of cardiotoxicity. A cardio-oncologist or a cardiologist with an interest in oncology should be involved to optimise them from a cardiovascular perspective. It’s about having an open discussion with the oncologist about their proposal and the additional risk and then feeding that back to the patient.
I tell patients this is a balancing act and we’re trying to ensure the benefit of cancer treatment outweighs cardiovascular risk. There are only few circumstances where it doesn’t and the risk of killing them with chemotherapy is higher than the risk of curing them.
People have different levels of acceptable risk and different priorities, so involving them in decisions is really important. My role is to facilitate safe cancer therapy.
Whenever we thought about cancer and the heart, we used to think about Herceptin and heart failure. Certainly, we do see left ventricular systolic dysfunction associated with Herceptin, but nowhere near as frequently as we used to because of all the cardio-oncology measures that are in place.
Occasionally we see patients with dramatic cardiotoxicity that develops as they’re receiving their cancer therapy. Classically, for example, someone who is receiving paclitaxel and acutely decompensates. They might get chest pain or go into heart failure, but, actually, these patients tend to do well and it’s reversible so they recover as quickly as they deteriorated.
We see patients who have early cardiotoxicity in the days and weeks following systemic anti-cancer therapy, or we can see it months or years later. There’s a challenge there to unpick the timeline and work out what’s causing what. Certain drugs are good at causing late effects and other drugs cause acute cardiotoxicity.
I learn all the time, as cancer therapies develop, about new cardiovascular toxicities or new presentations of cardiotoxicity. It’s really important to keep an open mind when looking after patients who have cardiac issues and have received, or are receiving, systemic anti-cancer therapies and think could these two things be related.
Not only do we see a spectrum in terms of the timeline, we see a spectrum with the cardiotoxicity and the clinical presentation.
Hypertension is hugely under-recognised and under-treated in cancer patients. We know there’s a lot of overlap between risk factors for cancer and for cardiovascular disease, so it’s not surprising that many cancer patients have hypertension. If you add in that they’ve been diagnosed with cancer; they’re likely to be undergoing challenging, difficult-to-tolerate treatments; and they’re anxious, frightened, in pain, anaemic and tired – all these will raise their blood pressure.
Then you’re giving them cancer drugs, many of which can cause hypertension. We can see why 40% of patients with cancer have hypertension. But we undertreat it and we excuse it, but we should be managing it like we would with any other patient with hypertension. We should be monitoring them appropriately and getting them on antihypertensive medications.
Vascular toxicity tends to be more of an issue with the treatments we use for gastrointestinal cancers. We see destabilisation of coronary artery plaques, patients presenting with myocardial infarction, patients with coronary artery spasm. This may just be a bit of indigestion-type chest pain, and the patient may not even present to a healthcare professional but, at the other end of that spectrum, we’ve seen patients with coronary artery spasm who’ve presented with a cardiac arrest. It’s really important that these patients are taken seriously when they say they’ve got chest pain and that everyone is aware that it is a recognised vascular toxicity of the these chemotherapeutic agents.
Myocarditis is an increasing worry with patients who receive checkpoint inhibitors or immunotherapy. When I started in cardio-oncology, we only used immunotherapy for patients with melanoma or renal cell carcinoma. Now, around 75% of cancer patients are eligible for treatment with immunotherapy and that’s not just in a palliative setting, but in a neoadjuvant and adjuvant setting.
Checkpoint inhibitors have a 2% risk of causing myocarditis, and if we miss this and don’t treat it, the patient will die from the myocarditis. So, it’s really important that we consider this in patients who have received immunotherapy because they may present innocuously with fatigue, ankle swelling, breathlessness. You can see there’s a challenge there because what cancer patient doesn’t have those symptoms? But keep it in mind for these patients because the sooner you recognise it, the better the outcome for the patient.
But it’s not all about cardiac function. We need to consider blood pressure, QT interval, myocarditis and, thinking about later effects, things like ischemic heart disease.
Patients who had radiotherapy, particularly mediastinal or left-sided radiotherapy, many years ago can present years down the line with quite significant proximal coronary artery stenoses. And it never fails to amaze me how many patients forget to tell you they’ve had cancer when you’re taking their history. I think a lot of patients block it from their memory. So, particularly if a younger patient is presenting with angina-type symptoms and you think it’s a bit unusual, always ask about their cancer history specifically.
I always say to the oncology teams regionally that cardiotoxicity is a bit of a jigsaw. Certainly, understanding the patient’s clinical presentation is key, and then using that with complementary biochemical and other cardiac investigations to fit everything together.
Within cardiology teams, we’re all familiar with troponin and NTproBNP, but remember, the oncology teams are not. It’s important we help them interpret these and have pathways in place so they know what to do with abnormalities.
Imaging, obviously, is our backbone of decision making within cardio-oncology. It’s really a complimentary modality to help us put into context the clinical signs and symptoms and the biochemistry. Where possible, we should be measuring global longitudinal strain (GLS) and ejection fraction using 3D volumes in all our oncology patients.
We know 3D volumes are more reproducible and have less temporal variability. We don’t want to stop someone’s chemotherapy because we think they’ve dropped the ejection fraction when, actually, it’s just because the pictures were a bit rubbish and the 2D ejection fraction was way off. We need to make sure we’ve got accurate, reproducible echo data to help guide our decision-making.
There’s been a real shift over the last decade in trying to continue chemotherapies and radiotherapies and permitting a certain degree of cardiotoxicity. It comes back to the question of at what point does the risk outweigh the benefit?
It’s about changing your mindset from ‘should this therapy be discontinued’ to ‘how can this therapy be continued’. Because we know that if we interrupt anti-cancer therapy, it’s an independent prognostic marker for worse disease-free survival and overall survival.
For the patients with asymptomatic cardiotoxicity or mild-to-moderate cardiotoxicity, the challenge is trying to work out what to do to safely continue their cancer therapies. And that’s where the cardio-oncology service comes in with increased screening, monitoring, optimising of cardio-protective therapies.
It’s important that if we stop therapies, we consider rechallenging them. In the past, nobody would be rechallenged, but many patients will have a successful rechallenge once they’ve been optimised from a cardiovascular perspective.
That brings us back to the multidisciplinary team (MDT) and the importance of that two-way discussion with cardiology and oncology in terms of making sure that we’ve thought about everything to enable treatment to continue or be restarted.
When you’ve got a cardiologist making unilateral decisions, you’re going to run into difficulties. A pivotal part of our service is that we see patients quickly. There’s no point a patient being referred with their chemotherapy discontinued and me saying I’ll see them in six months, which is probably what a lot of cardiology outpatient waiting times are. We’ve set our service up to see patients within two weeks. If we can’t see them within that timeframe then we offer advice on cardiovascular risk to ensure treatment interruptions are minimised.
Efficient communication throughout is important. I work at Liverpool Heart and Chest Hospital, four miles from Clatterbridge Cancer Centre. That’s a different Trust with a different electronic patient record. We can’t see each other’s echocardiograms, so it’s challenging, but we’ve worked hard to improve communications between the teams.
We’re becoming more aware that it’s not just thinking about a patient’s heart or cancer but thinking holistically because these patients are complex and we want to get the decisions right.
In terms of the MDT, people dip in and out, depending on the patient and the clinical need. We have cardiologists, a cardio-oncology specialist nurse, specialised cardiac physiologists, medical and clinical oncologists, palliative care specialist nurses, pharmacists, radiologists, surgeons, anaesthetists, a dietitian.
When I started the cardio-oncology service in 2019, we ran a fortnightly clinic, but now we run three per week. We have a weekly ward round at Clatterbridge Hospital, four weekly oncology echo lists and a weekly virtual MDT where we ensure we have oncology, cardiology and radiology representation as a minimum.
Everyone’s perspective is different, and it’s really important that we all understand these to ensure that patients have the right decisions made.
Momentum is increasing, and certainly people are more aware of it now than they were 10 years ago. There are not many fully fledged cardio-oncology services, but I think more and more cardiologists and oncologists are aware of cardiotoxicity and are seeing patients within their clinics and realising they need to funnel them in a slightly different way.
We’re all aware that the NHS has no money, so trying to develop business cases and set up new services is incredibly challenging, but one of the things that I feel really strongly about is if we set up those services and risk stratify patients to optimise them at baseline, we’re hugely reducing the risk of cardiotoxicity and saving money. The drugs that we use to treat cardiotoxicity cost tens of thousands of pounds per patient, and if we can reduce the risk of that happening in the first place, prevention is definitely better than cure. I think the service will pay for itself over time, but that is very challenging.
The challenge is getting enough people interested. Everyone is busy and when you go along to a TAVI operator or a cardiothoracic surgeon and say, ‘I want to get you interested in cardio-oncology’, people run the other way. But the service does speak for itself: the more patients we see, the more we assess cardiovascular risk, the less interruptions to systemic anti-cancer therapy are happening. The oncology team certainly values the service, and they are desperate for help with this group of patients.
So, in terms of tips for setting it up, get an interested oncologist on board and start your discussions there to build a joint business case.
In terms of funding, it took me four years to get funding for a cardio-oncology specialist nurse. The only way I managed in the end was getting our local Cancer Alliance to fund a year’s salary and a private donor contributing as well. We’re all facing challenges in the current climate and it’s about looking at alternative funding sources. So, could your local hospital charity do a funding campaign for you to help with these services?
With capacity, the way we’ve set the service up means these patients are all going through dedicated cardio-oncology clinics. We make sure we save urgent slots every week, but we drowned in patients after a couple of years and I suspect that as demand goes up again, we’ll struggle. But for now, we’ve just doubled the number of clinics that we offer, and having a cardio-oncology nurse specialist has further allowed us to increase our capacity.
7th January 2025
Significant strides are being taken in oncology, with treatment innovation and expanding skill sets supporting best practice among the multidisciplinary team. Speaking to Saša Janković, clinical and oncology pharmacist and ESOP president Professor Klaus Meier discusses the significant opportunities and challenges in the field and how collaboration is the key to an even brighter future.
‘Oncology works better when we give more power to pharmacists’, says Professor Klaus Meier. It’s a bold but thought-provoking statement, and he practices what he preaches.
As president of the European Society of Oncology Pharmacy (ESOP Global) – the world’s largest multinational oncology pharmacy organisation, founded in 2000 in Prague, and now with a membership of more than 4,500 members from 76 countries – Professor Meier is at the forefront of addressing challenges and harnessing opportunities in this critical specialism. Ultimately, he is a champion of the integration of oncology pharmacy into multidisciplinary clinical practice.
The specialism of oncology pharmacy has grown significantly across Europe since ESOP’s foundation, but the heterogeneity of healthcare systems across the continent remains a key challenge. ‘Every country in the EU has its own responsibility for education, health and related matters, which makes it difficult to implement universal programmes,’ Professor Meier notes.
To address this, ESOP has dedicated efforts to establish standardised education and training for the sector. A full member of the European Cancer Organisation, one of ESOP’s landmark initiatives is the European Certification Program for Oncology Pharmacy (EUSOP) – a comprehensive 100-hour initiative combining e-learning, an international workshop, and national training sessions, with participants achieving the title of European Oncology Pharmacist upon completion to signal their specialised expertise.
‘We aim to give pharmacists the tools they need to contribute meaningfully to cancer care,’ says Professor Meier, ‘and this structured approach underscores the specialism’s role within the broader hospital pharmacy framework, as well as its pivotal contribution to multidisciplinary cancer care teams.’
While medicines shortages and supply issues continue to make headlines across Europe, Professor Meier says one of the most destabilising additional challenges for the oncology pharmacy sector is war and conflict.
‘Much of the work ESOP does is about giving pharmacists the opportunity to come together because we cannot be helpful when we are not full of knowledge, but current conflict situations across the world are hampering these efforts,’ he says. ‘For example, when ESOP started, we initiated an exchange with Russia and Ukraine, and we are waiting for the right moment to pick the personal local exchange up again.’
Further hurdles include the disparity in drug availability across Europe. ‘In some EU countries up to 50% of European Medicines Agency-approved drugs are not available due to governmental or insurance-related barriers, and this impacts not only patients but also clinicians who miss the chance to become familiar with these therapies,’ he says.
ESOP is therefore actively lobbying for cohesive pharmaceutical legislation to ensure equitable access to essential drugs across all EU nations. Its working groups – comprising members from Asia, South America, Europe and Africa – regularly convene to share expertise and develop solutions to dive these efforts forwards.
On the positive side, technological and scientific advancements are reshaping the oncology pharmacy landscape and optimising the care that healthcare professionals can offer patients.
‘Developments such as mRNA cancer vaccines, pharmacogenomics and personalised medicine are going to be transformative for the sector,’ Professor Meier says. And he draws a parallel to the Covid-19 pandemic when pharmacists played a central role when carrying out vaccinations, even in countries where this was previously unprecedented.
‘At the start of the pandemic we knew very little about Covid-19, there was no vaccine, only panic,’ he recalls. ‘But then our pharmacists began to be included in the vaccination programmes in countries where they never have been included before, such as France, but also in community pharmacies, which before had only offered flu vaccinations – like in Germany where it had been unthinkable that pharmacists would do that, as only doctors had the allowance.’
Despite the positive learnings from and progress made during the pandemic, there’s still a long way to go to consolidate pharmacists’ roles, responsibilities and skills across the continent. Professor Meier therefore encourages pharmacists to learn from colleagues and each other and keep an ear to the ground to ensure they are ready for future change.
‘If you made a map of nations, it’s clear that how healthcare is delivered in one is not necessarily how it is done in others, but these advancements underscore the critical need for pharmacists to stay at the cutting edge of innovation and to adapt rapidly,’ he asserts.
To support this adaptation, ESOP places a strong emphasis on education. Its flagship event, the European Congress of Oncology Pharmacy (ECOP), was held in October 2024 in Lisbon and aimed to offer pharmacists a balance of expert scientific content and hands-on guidance.
‘This year’s Congress had high-level lectures about scientific questions, as well as covering a lot of practical training issues, such as how to write a clinical case at the end of the 100-hour education programme,’ explains Professor Meier. ‘We try to make the Congress a useful tool for attendees because when you have no tool you cannot open a box, so we are working on giving everybody the tools to express their knowledge and make collaborative connections with others – and I think we succeeded this year. In fact, I don’t think we’ve ever had a Congress with such positive feedback afterwards.’
Despite largely being aimed at oncology pharmacists, a welcome cohort of community pharmacy colleagues also participated in the Congress. Professor Meier says it’s important to acknowledge that the treatment and care of cancer patients is much broader than just the therapies provided in clinics, partly due to the success of these therapies. The subsequent reduction in morbidity increases the number of chronic patients that need long-term support, which can be provided in a variety of settings. As such, Professor Meier is clear that the community pharmacy sector plays a key role in maintaining joined up working and best practice across cancer care.
For example, a pioneering ESOP initiative in Germany seeks to engage the country’s 18,000 community pharmacies in supporting oncology patients.
The Oral Cancer Therapy Initiative provides community and hospital pharmacists with essential information about cancer drugs, side effects and adherence, while patients are given tools to document their experiences and facilitate better communication with the healthcare professionals they come into contact with throughout the system.
‘Community pharmacists can give patients a plan that makes sure they are able to take their cancer drugs in the right way at the right time,’ says Professor Meier. ‘If after two or three days they are having side effects, the patient can go back to the community pharmacist and check they have taken the drug in the right way, and if they still feel bad the pharmacist can make an immediate appointment with their doctor to check if what is prescribed is the right concentration or not – positioning community pharmacists as the coordinator between the patient and the doctor.’
Available in 10 languages, the initiative has recently been rolled out in Poland, and it is also due to commence in Hungary in 2025. A working group is poised to ensure it is then implemented further afield. And for pharmacies that are not yet equipped for its full application, the ESOP website offers a scaled down version with basic information in English that each country can translate into its own language.
Dismantling barriers to cancer care is a recurring theme in Professor Meier’s vision and this was his key message at the ECOP Congress.
‘As pharmacists, we are part of the multi-professional action that enables comprehensive care for patients,’ he says. ‘It is not drugs that is the measure of all things, but the implementation culture with the direct involvement of the other players in the healthcare system and, above all, the patients.’
ESOP’s quality standards, first published in 1996, serve as a foundation for such collaboration. These essential requirements for best practice, now in their seventh iteration as QuapoS 7 and translated into 23 languages, cover everything from aseptic preparation to drug administration and education and, according to Professor Meier, ‘enable oncology pharmacists to work confidently and collaboratively, whether they are in Bulgaria, South Africa or Mexico’.
Looking ahead, Professor Meier is optimistic about the future and the ability for pharmacists to proactively support progress in oncology. He concludes: ‘Our goal will continue to be to empower oncology pharmacists to use their knowledge to its fullest potential, embracing advancements in technology and personalised medicine, while continuing to advocate for patients in the face of systemic challenges, so oncology pharmacists can make even greater strides in optimising cancer care.’
4th December 2024
Our latest Clinical Excellence in Respiratory Care event included a fascinating panel discussion on managing pulmonary hypertension as a multidisciplinary team (MDT). Here, you have exclusive access to the session recording.
Hospital Healthcare Europe and Hospital Pharmacy Europe editor Helena Beer was delighted to be joined by three specialist clinicians for this panel discussion: David Kiely, consultant respiratory physician and professor of pulmonary vascular medicine at Sheffield Teaching Hospitals NHS Foundation Trust, alongside Colm McCabe, respiratory consultant in pulmonary hypertension, and Heba Nashat, consultant cardiologist in pulmonary hypertension – both at the Royal Brompton Hospital in London.
Recorded on 21 November 2024 and shared at the Clinical Excellence event the following week, the panellists discussed diagnostic challenges and the role of imaging technology in pulmonary hypertension, integrating cardiology and pulmonology treatment plans and how to best work together as an MDT for the benefit of the patient. Scroll down to watch now.
Watch more Clinical Excellence event sessions via our new Clinical Excellence Catch-up zone.
You can find brand new interviews and case studies, plus round ups of previous Clinical Excellence event sessions and much more in our Respiratory zone – just look out for the orange Clinical Excellence tag to read a whole host of content that can help to inspire your practice.
We’ve recently started work on 2025 content and there are some brilliant pieces coming through the pipeline so remember to check back regularly so you don’t miss out.
If you haven’t already, you can also sign up to our newsletters, which will bring all our latest content, including Clinical Excellence straight to your inbox each week.
View the agendas and register for our next Clinical Excellence in Respiratory Care and Clinical Excellence in Cardiovascular Care events now.
20th June 2024
Speaking at Hospital Healthcare Europe’s Clinical Excellence in Respiratory Care event, our panel of three experts considered the role of critical care in respiratory medicine. Dr Andrew Chadwick, Jane Scullion and Dr Phyllis Murphie PhD discussed how guidelines and best practice for treating respiratory patients in critical care have changed since the advent of non-invasive ventilation, as well as the lasting impact of the Covid-19 pandemic in this field.
With increasing use of non-invasive ventilation over the past 30 years, not to mention the wide-reaching impact of the Covid-19 pandemic, respiratory critical care has seen its fair share of change in best practice, which three Clinical Excellence event panellists know all too well.
Dr Andrew Chadwick is a respiratory and critical care consultant at Oxford University Hospital NHS Trust, where he is part of the nationally recognised special airways clinic. He reviews over 300 severe asthma, chronic cough and complex breathlessness patients each year and has a vast experience in critical care, as well as a self-professed love of non-invasive ventilation (NIV).
Independent consultant respiratory nurse Jane Scullion spent many years working at Glenfield Hospital, part of the University Hospitals of Leicester NHS Trust, across the TB service, asthma clinics, COPD services and interstitial lung disease clinics. In her early career, she was heavily involved in critical care and NIV on respiratory wards and, more recently, has worked in medical negligence and long Covid clinics.
Dr Phyllis Murphie PhD is an independent respiratory nurse consultant working in Dumfries and Galloway in Scotland. She specialises in sleep medicine and NIV, having introduced the NIV service into her hospital many years ago. In 2020, she led a respiratory nursing team through the Covid-19 pandemic and introduced changes to ensure the effective delivery of respiratory care during this challenging time.
Chaired by Garry McDonald, respiratory pharmacist at University Hospital Crosshouse in Scotland, the panel consider the trajectory of guidelines for managing respiratory failure, their take on ensuring patients respond well to NIV, and key learnings from the Covid-19 pandemic that are still in use at their hospitals today – including the somewhat divisive proning technique.
Dr Chadwick: It is fascinating how our mechanisms of treating respiratory failure have changed or haven’t changed and how we got here. If we wind our brains back, NIV starts getting developed in the late 80s and it starts to be used at home. It was late in the 90s when it exploded onto the scene in hospitals, with the work out of Leeds from Plant showing the staggering effect on COPD exacerbations. Number needed to treat: three to save a life. This is eye watering, it’s fabulous.
Then there is a real push in the early noughties of how far can we push these machines? What can we do? What works and what doesn’t? Then, you start getting a mixed signal in true acute respiratory failure. The narrative starts becoming: are we overusing this? Are we delaying intubation? Are we holding back, holding the patient in a period of risk?
There was a bit of controversy about it, and that built. Then, in 2020, a systematic review in JAMA pointed out that there are lots of small studies, put them all together, and what you start getting is a real signal of benefit of delaying intubating, a signal of benefit of saving life, and that helps us go forward.
Then Covid comes. A real challenge came out about are we sure we have got this right? And you had University College London at the start of this – we copied them in Oxford – saying, let’s hold people on continuous positive airway pressure (CPAP), let’s try and avoid incubating. Partly because we were worried we were running out of oxygen and I’m sure a lot of other places were too. And then the trial showed that this really does help to avoid intubating.
And now I think we’re left in a real limbo. I hope that gives a sense of where we are now, as I think where we are now is really interesting. If you were to go into the Berlin ARDS definition of guidelines, I think you’d find NIV spoken of in relatively negative terms in respiratory failure, apart from COPD, pulmonary oedema and cardiogenic shock. But I actually think in the real world, with really good data that I’ve hopefully just pinpointed, you find a much more balanced view and indeed a view that’s increasingly going: I wonder if we should hold, hold, hold off intubation.
If you look at the COPD literature, NIV to treat is unbelievably good compared to almost anything else in medicine. Initial trials using a pH of around 7.3 show significant improvements. I think in treating acute respiratory failure, increasingly, NIV can be a really good adjunct. Intubation is clearly the end of the road.
Ms Scullion: I remember the great big machines that you couldn’t carry around, trying to prop them up next to patients to ventilate them to start with. Now, we have lots of small portable devices, not just in a hospital setting, but people with longer-term conditions are also managing at home, especially with the LSAs [Lung Support Assistants] travelling with it.
It has been life transformative. Things do evolve. The nicest thing that came out of Covid was that respiratory proved that we could do the research, could look after the patients and could get results out of it that will alter as time goes on. We didn’t have enough critical care beds and the ordinary nurses with no respiratory background stepped up and did this, as did the doctors on the wards and pharmacists and physios. We ran almost mini high-dependency units wherever we could run them.
Dr Murphie: It’s remarkable, the evolution of the non-invasive ventilation story. From the days of my first job, I came to the consultant, who had two NIV devices in a cupboard and he didn’t know what to do with them. So, he said, ‘Phyllis, do you think you could arrange some training’, so it started from there.
When these devices were retired, we moved up to the next version, and then the next version, so the whole evolution of the devices that we used in secondary care, particularly during Covid, was a very steep learning curve for a lot of people because we had to do this outside of critical care in the wards.
Covid was the beginning of respiratory support units evolving. We spent hours and hours training all the staff to come in and be able to manage ward-based CPAP and NIV quite safely. For me, NIV really came of age at that point in time in terms of people’s understanding of it, when to use it and when not to use it.
Ms Scullion: It is really difficult when you have a patient in extremis, and you are going to put something onto the face. It is difficult when patients are really ill – you have to have a lot of time to get them to accept things.
I have often thought that as part of pulmonary rehab, especially with COPD patients, we should take these things in before people need them so they can see them and get used to it and feel what it is like because you can’t make a rational decision when you are extremely ill. We know some patients won’t tolerate it although a lot do. It can be uncomfortable, it dries your mucosa and there are all the other side effects. So, it is time and patience.
Dr Murphie: Something that is really important is knowing how to mask fit properly. Being able to make sure they have got the right size mask on, because then you start getting pressure sores and things on the nose. Fitting the masks and making sure that people know how to fit them properly and not do harm is a really important skill to learn as well.
It takes a certain level of skill to acclimatise your patient onto the therapy. You have to be patient, work out the fears and talk them through it. Sometimes, you have to start with the sub-optimal measures to get them comfortable and confident enough to wear the mask. You give them reassurance that this is something that works really well; it could shorten the length of time they are in the hospital and make the other therapies work better as well.
Dr Chadwick: This is one of those times when you really need to add in all your confidence, and you need to get the patient to buy into it. Don’t underestimate the power of reassurance and the power we have as healthcare professionals to do that.
So, coming in, being reassuring and then asking for one hour of NIV and really trying it. Then you can judge the blood gas, and you can go back to them and their family, and say, ‘look, we really tried, but we’re not winning with this, so let’s not’.
Or you can say, ‘Actually, look, we have really made a big progress; our pH has jumped from 7.1 to 7.15, so that’s a huge difference’. Then suddenly, you are in a new conversation saying, ‘well, actually, I’ve got physiological proof that this works for you, so work with me. This is going to be brilliant’.
Dr Murphie: Having outreach teams is really important. Making sure that we can talk to the teams in, say, the combined assessment unit. They want to see patients early. If they are starting to struggle, and you can see that their blood gases are going off, then we want to know early on.
Getting in early and trying to work with the patient to reassure them that there is something worth trying to see if they can feel better. They can turn a clock back very quickly and start to see improvements if it is applied early enough and not too late.
Dr Chadwick: You just jumped onto one of my pet peeves. Number needed to treat is unbelievably good – better than almost everything else in medicine. Increasingly, what you are seeing is drift in all of us, in every clinical practice, we are just holding it back later when the patient is sicker. Early is better. You get in there early and stop the hypercapnia, if that’s what you’re doing with NIV. It’s much easier than coming in when they are really down the line. It’s a real pet peeve of mine: what are we doing holding back? There is a kind of odd culture of holding NIV back.
Ms Scullion: The acceptance of patients is better if they are not confused and not fighting it and not agitated and not desperately ill. That has to be the best option to do it as soon as possible.
We want to do the best for the patients in front of us, and sometimes NIV is the best treatment that you can give, and it stops a lot of other things. Our patients nowadays do get fully ventilated and do get off ventilators, but not in great numbers.
A lot of them do poorly, and it is not a terribly nice prognosis at the end for the family to cope with. So, NIV, for me, is a nicer option because the patient is still in the room with their loved ones.
Ms Scullion: I was so proud of the respiratory community during Covid because we had to get on with it. A lot of the decisions were made by clinicians. We did for patients what we could and everybody – across the board, pharmacists, physios, put their shoulder to the wheel and did it. Even in patients when we were proning, and things like that, you know there were 10 or 12 people proning a patient.
I mean, proning was something where, if you can say, good came out of Covid because it worked. It’s probably quite an old technique. I’ve seen the pictures of the old machines where people were turned in the machines and had a mirror so they could see up or a mirror that could see down. So, it has been around for a long time and often, just because it’s old, doesn’t mean it’s not good.
Dr Chadwick: It is perceived as an old trial. I was working out in Paris briefly on a long placement and they, honestly, were flabbergasted pre-Covid that we don’t just prone everybody. And in England, the problem is – and this is common across units and there’s no judgement because these are world-class British units – but we would always say things like, ‘oh, it’s not safe, you might dislodge, you might do something’. And you’re absolutely right, Covid put that all to the wind.
Work done by people like UCL really nudged the needle back to say, ‘come on now, prone them, it really works. It buys you space to ventilate them kindly and keep within those safe parameters’. So, you’re absolutely right, Jane, it’s another fabulous example of where real positives came out of Covid and essentially just reset that needle and how we treat acute respiratory failure.
Dr Murphie: We had the Army logistics teams come in and they basically organised and changed the flow of the hospital. Dumfries and Galloway is a brand new hospital with all single rooms, which was fantastic. The air changes in each single room six times per hour, so we actually really didn’t have a huge amount of in hospital transmission.
We moved the respiratory ward right along to the other end of the hospital so we were very close to the combined assessment unit. When patients were being moved, there was a green flow and we had a red flow. The green flow was the clean way to go and the red for the contaminated way. So, that really changed the way in which we actually managed patients in the hospital and we had a command control structure that did work really, really well in that environment and it served a purpose at that particular time and helped us to think about how we carry on and give safe care in the really, really difficult place that we were all working.
And that brings me to the point about MDT working. It was fantastic. Every morning, at nine o’clock on the ward, we would have a huddle and every single discipline was there to actually be involved in everything that we needed to do that day with the patients.
For me, the shining stars were the physios and the occupational therapists (OTs). They were so good at trying to get people on their feet. Anybody who had been in critical care and had been ventilated, they’d lost so much of their muscle tone, health, you name it. And the physios and the OTs got them back on their feet and got them home again and it really did shine a light on how great our MDT colleagues are.
Dr Chadwick: We had loads of colleagues, like our vascular surgeons, who came and said, ‘we’re here to help’. The way our respiratory MDT started setting up was that we gave them a physio to lead them as a proning team. There’s this wonderful image of Annika who’s an amazing physio and quite a petite lady, and these six quite bulky vascular surgeons turning this patient. But they learned very quickly that the rules were you just do what Anika says to the letter. It was serious because you’re turning someone on a ventilator – you can really muck it up – but it was really wonderful to watch.
Exactly as you describe, Phyllis, it was fabulous MDT working. And that’s actually stayed with us in Oxford: to this day: we do a lot more proning and our physios still run our proning teams, not our doctors. We’ve decided that they do a better job, and therefore that’s very much left with them. Whoever’s there doing the proning, be that a consultant or whoever it is, that doesn’t matter as in that moment, the physio is in charge. We listen to them, we do what we’re told and we prone very safely.
I think acute respiratory failure is just a lovely example of a bit of medicine where the MDT does make it all work. If you took any one cog away, all of it falls away.
7th June 2024
Speaking at Hospital Healthcare Europe’s Clinical Excellence in Respiratory Care event, our panel of three respiratory experts considered the use of diagnostic imaging for respiratory conditions. Dr Uta Hill, Jane Scullion and Dr Zaheer Mangera shared their views on how imaging techniques such as chest X-rays, CT scans and MRI can support the diagnosis and staging of respiratory diseases; the challenges that are being faced by the multidisciplinary team; and how new innovations are set to revolutionise patient care.
The diagnosis of respiratory conditions is reliant on a whole host of imaging techniques, holistic approaches and multidisciplinary input, all of which formed the basis of an in-depth discussion between three Clinical Excellence event panellists and chair John Dickinson, professor in sport and exercise sciences and head of the exercise respiratory clinic at the University of Kent.
Dr Uta Hill is a respiratory consultant at the Cambridge Centre for Lung Infection, part of the Royal Papworth Hospital NHS Foundation Trust. She is the clinical lead for cystic fibrosis and bronchiectasis co-clinical lead, contributing to the cystic fibrosis service and the hospital’s lung defence and immunology services.
Independent consultant respiratory nurse Jane Scullion spent many years working at Glenfield Hospital, part of the University Hospitals of Leicester NHS Trust, across the TB service, asthma clinics, COPD services and interstitial lung disease clinics. More recently, she has worked in medical negligence and long Covid clinics.
Dr Zaheer Mangera is a respiratory consultant and lung cancer lead at North Middlesex University Hospital NHS Trust who says he finds himself ‘knee deep in reviewing images’ every day as part of the image-heavy lung cancer pathway. He also works to support patients in quitting smoking and is involved in several tobacco dependency projects at a national level.
Together, the trio discuss how to successfully navigate imaging in diagnosing and staging respiratory diseases and the ways in which clinicians and the wider multidisciplinary team can support improved image interpretation, ultimate diagnostic capabilities and patient care.
Dr Hill: In my particular area, focusing on lung infection, we use CT scans a lot. That helps us to make the diagnosis of bronchiectasis, which is a structural lung disease predisposing to infections.
We then use CT scanning to assess the distribution of the bronchiectasis, the severity of the disease and the extent. This really helps us when we discuss it in our multidisciplinary meetings to plan how we treat these patients, how we manage them, where to target chest physiotherapy, which antibiotics or antifungals to use, and what other aetiologies might be necessary to assess further.
We are now able to use the low-radiation CT scans, particularly in my area of looking at lung infection. We can use that quite successfully to diagnose bronchiectasis. It does reduce the radiation exposure somewhat to the patients, which then may make it more acceptable.
Ms Scullion: As a nurse, I’m only allowed to do chest X-rays and CTs, although we do look at PET scans, MRI, and VQ scans. Working in interstitial lung disease is very multidisciplinary. We get a lot of chest X-ray abnormalities that are reported in, and we rely quite heavily on CT scans for diagnostics but also follow-up and prognosis.
Part of our therapeutic range of giving antifibrotics relied quite heavily on getting the right diagnosis for the right patient because, depending on your aetiology, you’ve got different treatments, so it was a lot more important. More recently, we have been allowed to give things for progressive lung disease – a lot of what we see is progressive.
We do pick up a lot of lung abnormalities that are actually just lung abnormalities that the patient gets really stressed and worried about but aren’t serious, and we will pick up some cancers and everything else.
Dr Hill: A few things that we have not mentioned, for instance, ultrasound. So, the value of ultrasound in looking at pleural effusions, evaluating them and then targeting areas for biopsy through thoracoscopy.
Often, it is also necessary to think about different types of CTs. So, we often use CTs with expiratory and inspiratory imaging to look for dynamic airway collapse, which can be a cause of recurrent coughing and chest infections but may not necessarily lead to bronchiectasis. But you would only pick it up on a CT scan if you asked for the inspiratory and expiratory imaging.
We use magnetic resonance imaging to longitudinally track changes in patients who are younger and where we want to limit radiation exposure. And that is also a key point: we are aware of the risk of radiation to the patients and the consequences that it can have, and that we keep thinking of modalities or ways of ensuring we minimise the risks.
Dr Mangera: Thinking of the lung cancer pathway specifically, it usually starts off with a chest X-ray, usually requested in primary care or via the emergency department. An interesting question that we have to try to answer often is whether a patient should go directly to a CT scan of a chest and bypass the chest X-ray and that is becoming increasingly part of normal practice. Rather than delaying a patient’s diagnosis by wanting a chest X-ray first – that can be a straightforward process but sometimes it’s not – we are increasingly getting patients straight to CT scan where the index of suspicion for peripheral cancers is on the higher side and this is now reflected in national guidance as well.
It also reflects that fact that patients, as part of a lung cancer screening and targeted lung health checks, are going straight to CT scan so it doesn’t, make sense to introduce a chest X-ray step on a cancer pathway when that step doesn’t exist in a screening pathway.
In addition to the CT scan, that is very much the centre stone of everything that we do in the early part of the pathway, we also use nuclear medicine, PET-CT scan, which is an important test for staging someone’s scan.
For patients with lung cancer, we image pretty much every part of the body depending on the need. We will invariably use MRI when we are worried about disease of the brain or the spinal cord or bone disease, for example. We will employ ultrasound for image-guided biopsies, and so we tend to use a whole plethora of radiology techniques as part of the lung cancer pathway.
Dr Mangera: It’s very, very clear that there is a hierarchy of scans. If we think about the chest X-ray first, although it is a good test, there is lots of evidence about how you can pick up cancers even quite early on. The reality is that one in four lung cancers will have a normal chest X-ray – roughly speaking. Things will get missed, and there is a degree of human error with reporting.
I’ve seen what looks like quite obvious cancers being missed by very experienced radiographers. It does not happen very often, but we are looking at very subtle findings sometimes, and subtle findings can very easily trick you into looking normal, especially when you are sitting in a dark room looking at scans endlessly day after day. There is some scope for AI to assist reporting radiologists and radiographers. One way it might assist is it may focus the reporter’s attention into a particular area, for example. It’s not going to replace someone reporting, it’s not going to produce reports, but it will perhaps suggest that an area needs a closer look by the reporter.
Going onto CT scan, which is our bedrock. CT scan is fantastic at excluding cancer, it is not so good at confirming whether the abnormality that you are seeing is a lung cancer or not. I have seen all kinds of things that we thought would be a cancer coming back as not a cancer – typically infections. Even in the last month, I have told somebody they probably have a cancer, and it has come back as tuberculosis, for example.
More often than not, when you report a CT scan is showing cancer, particularly when there is a metastatic spread with mediastinal lymph node enlargement, for example, then it is very good at picking up lung cancers. But, every now and again, we find that we sometimes pick up diagnoses that are very good mimics of lung cancers.
And then, beyond a CT scan, the testing strategy is very much around what is going to get the staging of the cancer and PET-CT scan, invariably, is a tool for staging. It is uncommon that you need to go beyond a PET-CT scan to stage – except in the brain where an MRI scan will be superior to a PET scan in terms of staging.
Dr Hill: I want to emphasise the importance of using radiology in conjunction with histology; microbiology, in my case; and cytology to hone down the diagnosis. In all these areas of respiratory medicine, the diagnosis and further management are made in a multidisciplinary team discussion, where consultants, nurses, physiotherapists and radiologists come together to make the diagnosis and plan the management.
Ms Scullion: It is probably around the history taking, because that tends to give you an awful lot. I only can speak from interstitial lung disease, but we get a lot of referrals in from rheumatology, we get a lot from cardiology as well. I think it’s really important that we don’t all look at our specific bit, that we look more generally at what is going on with patients.
So, their history, especially if there is going to be a drug reaction within the lungs, how long have they been taking it? What came first, was it the drug or was the progressive disease already there? For me, the patient is really central to it. That is why we are all working in multidisciplinary teams because we all bring a little difference to it. We have always benefited from having pharmacists in our team as well, because they’re very good at all these weird drug reactions that are actually causing lung abnormalities.
Dr Mangera: A cornerstone of imaging is not the technique; it is the person reporting. If you do not have an experienced radiologist in thoracic radiology, then everything can look like a cancer, and everything will be reported as a cancer, or as interstitial lung disease, or an atypical infection, or whatever it may be. The recommendation will always be lung MDT.
This is an increasing problem, particularly in the UK setting, where there is a significant gap in the number of specialist radiologists, particularly thoracic radiologists. And with an ever-increasing market of outsourcing, where, in many Trusts, a large proportion of scans are reported outside of a Trust by private organisations. You may well get someone whose day job is a neuroradiologist, but at night they are doing thoracic radiology as part of trying to clear backlogs.
So, the thoracic radiology specialist within an MDT is key. But at the moment, I’m sure everyone is finding this, our MDTs are swamped with cases that are not being dismissed at the first round of reporting because of a lack of specialist thoracic radiologists reporting scans.
Dr Hill: As clinicians or nurses requesting imaging, we need to make sure we provide the relevant information. So, the smoking history, very brief details on whether they’re looking for infection and what the symptoms are, not just saying ‘coughing’ and as the only information. It really does help the radiographer or radiologists reporting if they do have all the relevant information on the request.
Dr Mangera: There are so many challenges for radiology because of the capacity issue of not having enough scanners and how far behind the UK is in terms of having physical infrastructure and the sheer number of scanners required for our population and the human resources required. We are far behind other countries like Germany and France, for example.
But in terms of day-to-day practice, I think incidental findings is the thing that’s choking our service more than anything. One thing we all have to remember is every time you perform a scan, you will find things that you are not looking for and are not relevant to the presenting complaint. What do you do with these things? And how do you know who has ownership of that problem?
With lung imaging, for example, we now get referrals from CT coronary angiograms and cardiac MRIs, we get referrals from our colorectal surgeons all the time because they do a CT chest everytime they do a CT colonogram for reasons that I still don’t fully understand. Patients coming in with any kind of complex trauma will have a CT head-to-toe – that’s the standard of care now. So, the sheer volume of incidental findings coming through the door has rocketed. While we have excellent national guidance on how to manage lung nodules, the sheer volume is causing a significant amount of pressure on the service.
That is before we even go into how that affects patients. Telling a patient that you have a lung abnormality, which is very unlikely to be a cancer but could be a cancer, depending on the psychology of that patient, they will take it well, or it will keep them up at night, potentially, for the next one or two years whilst they are under that surveillance period. I think incidental findings are a real problem.
Ms Scullion: An issue that we have is needing scanners that will take our larger patients. We’ve got a lot of patients who are over the weight limit for a lot of the investigations that we want to do, although you could argue, is it limited the information you get back just because of the population size?
Also, in secondary care, we can over-investigate people an awful lot because we are able to. I think it is about knowing when to do the investigations and also when to stop doing the investigations and always backing it up in terms of what’s happening with the patient, their symptoms and the good clinical history as well.
It’s also about learning to offer that reassurance to patients that what may have sounded alarming to them, actually isn’t. Because we check progression of disease, a lot of people who have maybe had TB in the past or sarcoid can worry about it being there, but it’s inactive and it’s just left a scar. So, scarring to patient may be very worrying, but to us it’s resolved.
Dr Mangera: If you speak to radiologists, AI is the big thing. The message is AI is not going to be the death of radiologists. The way AI work is it will help to support radiographers and radiologists in identifying problem areas. Specifically, AI can report dozens and dozens of scans within minutes and so what it will do is it will allow abnormal scans to be put to the front of the queue for a radiologist to report.
I think where the big value in AI will be is if a patient has a potential abnormality that warrants an earlier review, they’ll be pushed right to the front of the queue. That will make a huge difference in the current climate where, in some areas in the UK, patients and doctors are waiting weeks and weeks for the reports to come back. With the rollout of AI, hopefully we’ll be reassured that those with probable abnormal scans are going to have their scans reviewed much earlier on. That’s what I’m told about the added value of AI over the next five years.
6th June 2024
Speaking at Hospital Healthcare Europe’s Clinical Excellence in Cardiovascular Care event, our panel of three cardiologists considered the role of multidisciplinary teams (MDTs) in cardiac care. Dr Tim Lockie, Dr Clare Appleby and Dr Shazia Hussain shared their views on how MDTs and their collaborative meetings can be most effectively managed.
The way in which care for cardiac patients is managed has changed dramatically in the last twenty years. Previously under the care of a single cardiologist, patients are now looked after by multiple specialists, including nurse practitioners, physiologists and various cardiac experts from interventionalists to radiologists and beyond.
With advances in cardiac imaging and new technologies, more diagnostic tools are available, and greater expertise is needed to interpret the findings. A range of healthcare professionals work together to determine the patient pathway through treatment and care, making multidisciplinary team (MDT) working a key component of contemporary patient care.
All three Clinical Excellence event panellists are heavily involved in MDT working across different areas, including coronary MDTs, structural MDTs and ward work, as well as applying the principles to NHS boardrooms.
They are Dr Tim Lockie, a consultant cardiologist and clinical service lead for cardiology at the Royal Free London NHS Foundation Trust; Dr Clare Appleby, a senior consultant interventionalist and clinical lead for intervention at Liverpool Heart and Chest Hospital; and Dr Shazia Hussain, a consultant interventional cardiologist at Glenfield Hospital, University Hospitals of Leicester NHS Trust.
Along with panel chair Rebecca Dobson, consultant cardiologist specialising in imaging and cardio-oncology at Liverpool Heart and Chest Hospital and the Clatterbridge Cancer Centre, they discuss how to successfully navigate the shared decision-making processes at the heart of MDT care based on their own experiences in clinical practice. As Dr Lockie said: ‘Multidisciplinary team working is what we all strive for. It’s not easy, and it’s not always well done. It has to be really nurtured and cherished.’
Dr Appleby: TAVI [transcatheter aortic valve implantation] is quite a big team. Within the hospital setting, there are the operators, but germane to the team are the nurse specialists who really run our service. They’re extremely important in terms of their role liaising with patients and referring hospitals and GPs in terms of investigations and managing patients’ and relatives’ expectations.
We have a TAVI coordinator who is an administrator, and that’s crucial for any large service. We have the cath lab staff where we actually do the procedure so the operating team. Then we do a lot of work with the wider team, so the referring hospitals around the region, GPs with special interest as well are very important in terms of in diagnosing and then investigating these patients. You have the outpatient setting and then the more procedural setting within the hospital, but I think in terms of something like a TAVI service, it’s really absolutely reliant on having an efficient multidisciplinary function.
Dr Hussain: We all do ward work and there are various teams that are involved in ward decisions like the TAVI team and the coronary revascularisation team. But, on a day-to-day basis, we have the ward multidisciplinary teams, which involve discharge coordinators, input from occupational therapists, physiotherapists, nursing staff, sometimes from the social department and funding.
These are often MDTs for patients where there’s a certain issue that is difficult beyond the medical issue and we see that on a daily basis – maybe there are difficulties at home, safeguarding issues, patients that are difficult to discharge – the voice of every member of that team is as important as the medical voice and, of course, we like to get the patients and their relatives involved themselves.
Dr Appleby: I think it’s probably about the structures that you have. For example, if we take the cath lab, where you’ve got a very high-intensity environment with many different staff groups performing procedures. We have a system, which I think is common to many trusts now, where you try and avoid a hierarchy and you have a more horizontal approach.
We have things like halt processes, your safety checks that you go through. We have a very structured approach so that each member of the team within that environment has an opportunity during those safety procedures to say whether they have all the equipment that they need, whether they have anything they want to raise. We do that in a very structured format before the performance of each procedure.
That’s a very particular example to a procedural environment. And that’s very different if you’re in a medical decision-making meeting, or perhaps on a ward base having a patient’s best interest meeting where you might have a much broader audience. But, if you can, structure it to enable other voices to be heard and have the patient at the centre of everything.
Dr Hussain: What’s really made a huge difference to our coronary MDTs is the presence of a neutral chair who is strong, can direct, is well aware of the different parties within the room and allows everyone to have an opportunity.
Sometimes, MDTs can get quite tense between different parties and, at that point, what you need is a chair. We’ve got Gerry McCann, who is our head of imaging, as our chair so he has a neutral perspective and is able to guide the conversation and take everyone’s viewpoint into account and also then decide when it’s time to move on from a conversation. It’s important that any MDT has an atmosphere of safety where everyone can speak, but it is a difficult area to navigate.
Dr Lockie: At the Royal Free we talk about the triumvirate. That works really at every level, whether you’re doing a ward round, whether you’re making decisions on how you’re going to plan your cath lab lists or how you’re planning all your other services.
We very much believe in this triumvirate structure, which is the medical input, the nursing input and then the operations team. At every level within the hospital, from the smallest units within a ward up to the trust executive team, the leadership structure is very much spread between those three. Everyone has a seat at the table, and everyone has a very important voice. Everyone brings something different to the table as well.
Dr Appleby: In the structural MDT, we have the same chair all the way through, whereas on our daily revascularisation MDTs it’s chaired by the surgeon of the week. I think it depends on what environment you are talking about, but for many of the cardiac-specific MDT processes, there are key people involved.
So, for structural, you will have your nurse specialists, your imaging cardiologists, your structural surgeons, your structural cardiologists, so there are key people in the room. But then we will invite, for example, the referring physician to present that patient.
Most of our MDTs are done virtually so that people who are off-site referring patients in can present their patients and advocate for the patients. So, I think it does depend on the specifics of the MDT, but certainly, there will be a key skill mix – our core – who you have to call up for that meeting to run efficiently and to be making safe decisions for patients.
Dr Hussain: The point of the MDT is that it’s a whole group of people making decisions. Certainly, in our structural MDTs we won’t just have one imaging person or one cardiac radiologist, there’ll be two or three of them. And from the cardiac perspective of the interventionalist we’ll be looking at those things as well. So, it’s never a decision that’s based on one person’s expertise.
Again, if we talk about good chairing and safety within the team, you would expect that if one person is talking outside their expertise, then they will have the honesty to say, ‘actually, what I’d like is a second opinion on this because I don’t know the answer’, and that’s the whole point of the MDT.
Dr Lockie: Like all of these things, you need to put the patient back at the focus of everything. There are disagreements about how to approach a particular problem, and I think all of us need to try to put our prejudices and biases outside the room and just look very objectively at what’s going on.
Increasingly, certainly with angiograms, we have got other things we can use now so we can look at intravascular imaging and we use that frequently now to define things further, and we have non-invasive functional data that we can reference. I think the days of disagreeing over angiograms, thankfully, are gone. I think everyone has now bought into a much more objective assessment of the situation.
But disagreements do happen. It will happen in every single MDT because people don’t conform to small, neat boxes and there is almost an infinite number of variables. You need to disagree agreeably, and as long as you keep the emotions out of the room and keep it focused on a particular patient, then that’s the most important thing.
The other thing to remember is that the output from an MDT is also guidance. You can take the output from an MDT, but you shouldn’t feel necessarily obliged to go down one particular path. In medicine, it’s not a black-and-white world, we’re not talking about objective, clear decisions. As the consultant, if your instincts, or your judgement, and the patient’s are different to the output from the MDT, we mustn’t find ourselves going down a particular route that’s been pushed very heavily from an MDT.
We need to be guided by the MDT. You should have a good reason if you do make a decision that goes counter to it. But also, at the same time, they’re not the ones who are either going to operate on the patient, be speaking to the family or having to pick up the pieces afterwards. And I think we always need to remember that this is guidance, not orders and you can always deviate.
Dr Lockie: I think that most hospital committees have some sort of patient representative on the group and including them in the decision-making is increasingly important and potentially difficult to navigate. Patients have their own understanding of things, and they don’t necessarily see the bigger picture.
With all sorts of decisions about services, patients do need to be involved. But as an organisation, and as different members of the triumvirate, we need to understand how to work effectively with patients to get their voice heard, but also to allow services to be planned and difficult decisions to be made.
It’s so important to have people in the room who actually know the patient. We will, unless it’s a real emergency, defer the meeting until the referring doctor, or the person bringing that person forward, whose actually met them, seen them walk across the room, shaken their hand and spoken to the family can be there. Otherwise, you end up making these very, very complex and potentially life-changing decisions based just on an angiogram, or a set of blood results, and we know there’s so much more than that. You’re absolutely right, it has to be patient focused because what might be right for one person will be completely inappropriate for another.
Dr Appleby: I know of another trust when they are presenting on the aortic valve disease pathway, they have a photo of the patient just to try and keep focus because sometimes the revascularisation MDT can become a bit of a bun fight – a robust atmosphere. Sometimes we just need to bring back and focus on the patients.
I don’t think it’s feasible to have video links live with patients, you’re just not going to be able to get through the numbers. But if you have ways of bringing the patient into that environment, I think it can be quite helpful.
Dr Lockie: I think that everyone needs that to feel valued. Mutual respect and kindness – these are things that need to come from all the people in senior leadership positions and really emphasise that on a daily basis. It’s about clear communication, it’s about respect for others, it’s about allowing others to have their voice in the space. I think it’s up to all of us to remember that. We have to set a culture where we want to work and where we want our colleagues to feel valued. The knock-on benefit in terms of staff happiness, retention, the overall atmosphere of the team can be really transformed by the simple things.
As soon as you have an environment where people get intimidated or, when they do speak, they’re made to feel as if their point is either irrelevant or not valuable in any way, then people are much less likely to speak out again. Whether you’re talking about the micro unit down on a ward, discussing an individual patient, discussing patients in a meeting, sitting in a boardroom, or you’re presenting a business case, I think that culture really permeates.
On one level, a negative culture can be terrible in terms of staff morale and retention, and you end up with people getting stressed and burnt out, not coming to work. But on another level, we’ve also had situations where there are genuine patient safety concerns because you get to the stage where certain individuals are so unwilling to actually listen to what other people say that people then stop raising concerns. Then things get missed and that is the sign of a properly dysfunctional team.
We’ve got quite a good system at the Royal Free called ‘what matters to you’ – a sort of formalisation of the speaking up process. It starts off with an opportunity to submit feedback online and then they have sessions where they bring it all together and you then have a constructive output and you repeat the process. It’s been really effective in sorting out some of these team relationships and building the kind of the structure which we all seek to have.
If things aren’t good or the communication isn’t there and you don’t have mutual respect, kindness and opportunity for people to speak out, it’s important to speak to your organisation about doing something to change that.
Dr Appleby: We are in the process of moving to a new single point of access pathway in terms of aortic stenosis. As part of that, we’ve had to engage with the different members of the team, particularly our surgeons, and agreeing criteria for where we would, up to the point of referral, triage them direct to surgery versus to the cardiologist.
It’s about really engaging the key members first. So, you can agree criteria for which we will triage them. And then when you’ve worked out a provisional pathway, we then opened it up to the wider team for comments. Now we’ve signed it off, which wasn’t a single event, I’m now in the process of going to the region, through our various partner hospitals and taking it through the clinic cardiology clinical leads.
There’s always going to be people who don’t enjoy the change. You have to explain why it’s very necessary, why it’s going to happen and then try and engage people who are perhaps the biggest opponents in designing that so that they feel they have some ownership of it. Then it’s about engaging the whole team and getting feedback before you roll it out.
And it’s getting across to the team that it’s not going to be a one-stop shop where we introduce it and suddenly everything’s great. It’ll be an evolving process. Things will come out of the woodwork we hadn’t anticipated which we’re going to need to deal with. So, managing expectations is also quite a big part of that.
Dr Hussain: It’s great to be able to engage all the key stakeholders from the beginning, but sometimes you just can’t and then you’ve got to go ahead and do it in the best way you can. We’re not talking about utopia where everyone’s going to agree, but as long as you know that it’s in the best interest, you’ve got the majority of people on board and, of course, management and the data behind it, then ultimately, if it’s for patient benefit, then you just have to go ahead.
22nd May 2024
Penicillin allergy is a common concern in healthcare settings, impacting patient safety, antimicrobial stewardship and successful infection treatment. Gerry Hughes explores the epidemiology of penicillin allergy and its significance in the context of patient care, as well as the need for a system-wide and multidisciplinary approach to penicillin allergy delabelling.
Penicillin is commonly implicated in drug hypersensitivity. In developed countries, between five and 15% patients carry a penicillin allergy label, and patients receive a penicillin allergy label by their third birthday in approximately 75% of cases.
However, a large body of evidence suggests upwards of 90% of patients with a penicillin allergy label are not truly allergic. In England alone, 5.9% of the population are designated penicillin allergic, with an estimated 2.7 million of these being incorrectly labelled as such.
It is important for patients and patient carers to know that common antibiotic adverse events, such as upset stomach, vomiting or diarrhoea are not symptoms of penicillin allergy. The Gell and Coombes classification of drug hypersensitivity (see Table 1 below) provides a useful reference for assessing and diagnosing drug-induced allergic reactions.
Hypersensitivity reactions associated with penicillin are predominantly Type I (anaphylactic, immediate onset) or Type IV (cell-mediated, delayed skin reactions).
Given the prevalence and implications of incorrect penicillin allergy labels, it is important for healthcare professionals to understand the impact of this issue on the successful treatment of infections and the broader implications for antimicrobial stewardship (AMS).
Table 1: Gell and Coombes hypersensitivity classification*
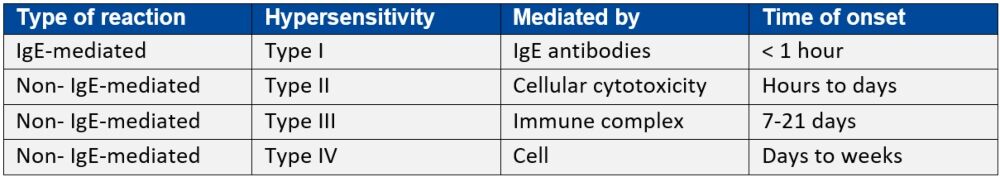
IgE: immunoglobulin E
*Adapted from Penicillin allergy: A practical guide for clinicians
Inappropriate labelling of penicillin allergy negatively impacts on patient care for several reasons. For many infections, penicillin, or beta-lactam antibiotics, are first choice therapy due to the weight of evidence behind their effectiveness.
Penicillin avoidance, due to a spurious penicillin allergy designation, can lead to use of less effective antibiotic regimens, unnecessary exposure to alternative antibiotic therapy, development of Clostridium difficile infection, additional time required in hospital and increased overall care costs.
The UK Health Security Agency’s ‘Start smart then focus’ AMS toolkit – first published in 2011 and most recently updated in September 2023 – makes evidence-based recommendations on effective AMS practices in hospitals, including appropriate management of penicillin allergy labels.
The toolkit advises secondary care clinicians and leaders about how local AMS policies and guidelines should include assessment of reported drug allergies and encourages them to conduct allergy delabelling where possible ‘to ensure patients are not denied access to the most effective therapy’.
Penicillin allergy assessment and delabelling (PAD) seeks to investigate a penicillin allergy history, remove the allergy designation where possible and/or desensitise the patient to future penicillin therapies.
Although immunology specialists are well-suited to develop and conduct PAD, it is a role often lacking in hospitals and other clinical environments. The British Society for Allergy and Clinical Immunology states that PAD performed by allergy specialists only, ‘cannot meet either current or future demand’.
Indeed, a recent study published in the Journal of Infection suggested that inaccurate penicillin allergy labels are magnified by insufficient allergy specialists. It investigated the feasibility of a direct oral penicillin challenge in delabelling low-risk patients with penicillin allergy by non-allergy healthcare professionals.
PAD is an evidence-based component of AMS and has previously demonstrated benefits in optimising antimicrobial choice and allowing for cost-effective treatment choices.
This evidence base extends to a multidisciplinary team (MDT) approach to PAD, which harnesses the expertise of infection specialists from medicine, pharmacy and nursing, amongst others.
An excellent example of this MDT strategy is the PAD toolkit developed by the Scottish Antimicrobial Prescribing Group (SAPG). This suite of resources is for non-allergy specialists to aid removal of penicillin allergy labels from patients with unverified allergic reactions, including resources for primary care practitioners and patients.
Professor Andrew Seaton, consultant in infectious diseases and general medicine at NHS Greater Glasgow and Clyde in Scotland, is chair of SAPG and recognises the importance of these guidelines in practice.
‘Whilst removing an allergy label sounds easy to do, it is difficult to do safely and sustainably without a formal structure,’ he says. ‘The SAPG toolkit has been a collaborative, multidisciplinary development which provides that structure and assurance for both clinicians and patients.’
St James’s Hospital (SJH) in Dublin, Ireland, provides such MDT-led PAD services, led by Professor Niall Conlon, consultant clinical immunologist and professor of clinical immunology at Trinity College Dublin.
Echoing Professor Seaton’s comments, Professor Conlon notes that successful PAD programmes require a systems-level approach. He underlines the need for an adequately resourced service, underpinned by commensurate education and training for the MDT involved. ‘A cohesive strategy is needed where all clinicians, not just allergy specialists, feel comfortable performing delabelling,’ he says.
In line with SAPG recommendations, SJH also provides resources to patients on penicillin allergy, including information on confirmed penicillin allergy.
Neil Powell, consultant antimicrobial pharmacist and associate director, antimicrobial stewardship at Royal Cornwall Hospitals NHS Trust in England, is the recipient of a clinical academic grant from the National Institute for Health and Care Research.
His research work focuses on removing erroneous penicillin allergy labels and leveraging an MDT approach to achieve that. He is currently developing a complex intervention that will facilitate PAD to be delivered by ward pharmacists and ward doctors.
Describing his work, he says: ‘This will enable penicillin allergy delabelling to be delivered to more patients across the hospital, with appropriate governance frameworks to ensure it is done safely.’
As part of Mr Powell’s research, a recent qualitative assessment of PAD facilitators and barriers found broad support among healthcare workers for an MDT approach. Driven by a desire to ensure patient safety, participants felt that PAD should be a multidisciplinary responsibility, shared between doctors and pharmacists and supported by pharmacy technicians and nurses.
However, the study also found that patient engagement and education on the topic was key. As with all healthcare interventions, participants also recognised the need for an evidence-based service, with sufficient resources to support its aims and objectives.
Mr Powell says: ‘From the qualitative studies I have done, healthcare workers have said that it needs to be a shared responsibility, not the responsibility of one specialty.’ In particular, he notes that ward pharmacists are well placed, as experts in medication safety and medication optimisation, to support and deliver PAD.
In fact, in September 2023, the Royal Pharmaceutical Society (RPS) launched a new checklist for pharmacists and pharmacy teams to help inform conversations with patients about penicillin allergy and determine their true status.
The checklist states that on admission to hospital pharmacy professionals should ensure allergy history is reviewed as part of the drug history and medicines reconciliation process. It also refers to guidance on setting up non-specialist allergy delabelling services in hospitals from the British Society of Allergy and Clinical Immunology.
PAD may also have a place beyond secondary care settings, and this goes further than simply providing education to primary care practitioners.
The RPS checklist, for instance, encourages GP practice pharmacy teams can run searches to identify any patients with a documented allergy that have since received penicillin and ensure their records are updated to reflect this.
The ALlergy AntiBiotics And Microbial resistAnce (ALABAMA) trial, based in the UK, aims to determine if a primary care penicillin allergy assessment package is safe and effective in improving patient health outcomes and antibiotic prescribing.
This multicentre, parallel-arm, open-label, randomised pragmatic trial aimed to recruit between 656 and 848 participants from participating NHS general practices in England. Recruitment was completed in 2023 and results are expected this coming autumn.
Recognising the potential to expand PAD to community settings, Professor Seaton highlights the continued need for collaboration. ‘If we can scale up [PAD] we can expand initiatives beyond secondary care and into the community. Undoubtedly a multidisciplinary approach is needed, [and] involvement of clinical pharmacists and specialist nurses will be crucial.’
It’s clear that penicillin allergy delabelling is an essential component of AMS, with important implications for patient care and public health. More work is required to educate healthcare professionals across sectors, as well as the public, on the prevalence and consequences of inaccurate penicillin allergy labels, but work is underway to support this across the UK and in Europe.
Ultimately, development of well-resourced MDTs and evidence-based guidelines will facilitate a safe and effective delabelling process, ensuring that patients receive the most appropriate treatment for their condition, all while minimising the risk of adverse reactions and keeping patients safe.
16th April 2024
From his base in Leeds, Dr Rani Khatib champions holistic, person-centred approaches and the collaborative power of the multidisciplinary team in cardiac care. Here, the newly appointed fellow of the European Society of Cardiology speaks to Allie Anderson about his innovative services that have enjoyed local, national and international acclaim, and how his own recent experiences as a patient have bolstered his professional work.
Dr Rani Khatib is a trailblazer in cardiology and cardiovascular pharmacy, achieving success locally and nationally through clinical work and research. As well as a consultant pharmacist in cardiology and cardiovascular research at Leeds Teaching Hospitals NHS Trust, he is visiting associate professor at the Leeds Institute for Cardiometabolic Medicine at the University of Leeds.
He has enjoyed acclaimed in Europe, too, sitting on cardiology allied professional groups for the European Society of Cardiology (ESC), and last year Dr Khatib was elected Fellow of the ESC in honour of his distinguished career.
‘It’s a huge recognition and becoming a Fellow of the ESC as a pharmacist rather than a cardiologist is an added bonus,’ Dr Khatib says. ‘The Society includes non-physicians as part of its structure because, simply, the care of patients with cardiology conditions requires input from multiple healthcare professionals.’
Dr Khatib embodies this multidisciplinary approach, not only contributing to but spearheading a number of pharmacy-led services at his Trust – and beyond. At their core are medicines optimisation and managing patient risk. ‘Cardiovascular disease is one of the biggest killers worldwide,’ he explains, ‘so there is a huge opportunity to ensure patients are on the right therapies and to optimise those therapies.’
Having noted suboptimal secondary prevention medicine (SPM) regimes and low adherence among myocardial infarction (MI) patients, Dr Khatib embarked on a project to ‘re-engineer’ post-MI care. Together with a consultant cardiologist, he established a post-MI multidisciplinary medicines optimisation clinic. Patients who had been hospitalised following an MI could see Dr Khatib, who is an independent prescriber, for a 30-minute consultation post-discharge to discuss any questions or problems they had with their medication.
He could manage patients autonomously but also escalate cases to the consultant cardiologist where necessary. ‘That was important because we worked together to identify the best set-up, so that we have access to each other, we work collaboratively, and we deliver what is best for the patients,’ Dr Khatib says.
Ahead of the clinic, patients were asked to complete a ‘My Experience of Taking Medicines’ questionnaire, known as MYMEDS. This self-reporting tool was designed to assess use of SMPs and to identify modifiable barriers – actual or perceived – to adherence. The completed questionnaire is a starting point for Dr Khatib to dig deeper.
‘It enabled patients to raise concerns about their medicines, whether that’s side effects or fitting medicines into their daily routine,’ he says. ‘Patients will often say “yes”, they remember to take their medicines, but if you have a further conversation using the MYMEDS tool, you might identify that they’re having problems swallowing the tablets so actually, they found taking them challenging.’ After identifying a barrier, Dr Khatib adds, he can work with the patient to overcome them.
The service was piloted between October 2015 and December 2016 among 270 patients. Optimisation of drugs improved significantly, with numbers of patients taking the recommended doses of ACE-inhibitors or ARBs increasing from 16.3% to 73.9%.
Patients reported significantly fewer concerns with their medications, non-adherence rates fell by up to 70.8% and readmission rates decreased.
In recent years, there has been a sharpened focus on holistic patient care and, with it, more emphasis on tackling multimorbidity. Patients with cardiovascular disease and type 2 diabetes have historically been managed by two distinct teams, but in Leeds, Dr Khatib spotted an opportunity to drive improvements in both specialties.
‘We identified that cardiology patients with type 2 diabetes were not necessarily receiving the best care,’ he says. ‘Newer diabetes medicines like SGLT2 inhibitors and GLP1 agonists also confer significant cardiovascular and renal benefits, so looking at the interplay between cardio-renal-metabolic seemed obvious.’
Dr Khatib established the CaReMe service, which streamlined cardio-renal-metabolic services into a ‘one-stop clinic’ for these comorbid patients. The consultant pharmacist-led clinic, supported by wider multidisciplinary teams, assesses patients six to eight weeks after an MI event.
It uses an adapted version of the MYMEDS tool – MYMEDS-Cardiometabolic – so as well as optimising medicines use and adherence, the consultant pharmacist provides a comprehensive review of the patient’s cardiovascular, diabetes and renal management needs. Such needs include key cardio-renal-metabolic biomarkers; analysis of risk factors; post-MI SPMs; and dietary, weight management and other lifestyle advice.
As well as improving patient outcomes, services like these highlight the crucial role of consultant pharmacists in multidisciplinary teams. They also create opportunities to expand input from appropriately trained senior pharmacists. Such initiatives free consultants to deliver other specialist services, thereby increasing capacity.
Moreover, Dr Khatib’s work has been taken further to not only reach patients in Leeds but nationally as well, notably with PCSK9 inhibitors. Designed to treat high cholesterol in patients who are not suitable for or poorly controlled on other lipid-lowering therapies, PCSK9 inhibitors are underused in optimising lipid management according to Dr Khatib.
‘We are always trying to improve access to innovative medicines, and bring what pharmacy can offer into the patient pathway to forward the cardiovascular agenda,’ he comments. ‘So, to improve access to these drugs, we set up another pharmacist-led, multidisciplinary clinic.’
Established in 2017, the clinic – the only service that was prescribing PCSK9 inhibitors in the Leeds area – also provides patient support, education and monitoring to promote adherence, as well as tackling statin intolerance.
The service proved successful and has yielded significant improvements in patients’ total and LDL cholesterol levels that are maintained at 12-month follow-up. It was deemed cost-effective and patient feedback was positive.
Furthermore, the project caught the attention of stakeholders at the Accelerated Access Collaborative (AAC), a UK-wide initiative aimed at extending access to high-quality healthcare, through improving uptake of the best treatments, for example. Harnessing his experience delivering the pharmacy-led service, Dr Khatib worked with NHS England and the AAC to develop a NICE-endorsed national lipid management pathway and the statin intolerance pathway.
‘Our model, uniquely, established a centralised service run by a consultant cardiology pharmacist and advanced cardiology pharmacists. We offered a vehicle for these medicines to be prescribed and demonstrated that lipid optimisation doesn’t have to be managed only by lipidologists,’ Dr Khatib explains. ‘We need to tap into the pharmacy profession more, and through collaboration with cardiology and lipidology colleagues the patient receives the best care, and the pharmacist is well-supported to deliver it.’
Dr Khatib believes that person-centred care must underpin every aspect of pharmacy. ‘As much as we talk about it, it’s often missed because it’s not as easy to apply as we think,’ he comments. However, being on the other side of the patient-clinician partnership has given Dr Khatib a broader understanding of the dynamics.
Having contracted Covid-19 in November 2020, he spent seven months in hospital in what he describes as ‘a terrible ordeal’ that caused multiple organ failures and cardiac arrests. This left him with extensive deconditioning and multiple morbidities – all of which he has documented in a Journal of Cardiac Failure editorial. His book with full reflections and lessons about this experience will soon be published.
‘I continue to live the patient experience and it has opened my eyes to a lot of things you only see as a patient, and not as a healthcare professional,’ he says, adding that it gives him a fresh perspective on patient need when it comes to multidisciplinary working.
‘Often patients said they preferred to see a cardiologist because they felt they’re more likely to get a rounded view, rather than just a medicines-focused discussion, which triggered something in my mind: we need to change the way we do pharmacy-led clinics to a more patient-centred approach,’ he explains.
This requires what Dr Khatib calls a ‘zoom out’ mindset, aided by tools like MYMEDS to support a holistic view. ‘So, when patients tell me about their experiences, I am ready to hear about their anxiety, their challenges going back to work, or how they’re getting on with lifestyle modifications,’ he comments. ‘I may not be able to solve those problems, but I can be considerate of them.’
In that way, Dr Khatib believes, pharmacy-led services can tick patient-centricity boxes while also helping to improve adherence and outcomes. He concludes: ‘I believe this is a better way of delivering the medicines optimisation concept.’
11th April 2024
Kicking off on 1 May 2024, Clinical Excellence in Respiratory Care is a one-day event for the multidisciplinary team exploring the latest advances in respiratory – and registration is now open.
Back for a second year, the Clinical Excellence events series brings together renowned experts from recognised Centres of Excellence and other UK and European hospitals to share their experiences of clinical innovations, examples of best practice and how they are improving patient care.
This year’s spring respiratory care offering has been developed by the team at Hospital Healthcare Europe and Hospital Pharmacy Europe with guidance from industry experts, including event chairs John Dickinson, professor in sport and exercise sciences, head of exercise respiratory clinic at the University of Kent, England, and Garry McDonald, respiratory pharmacist at University Hospital Crosshouse, Scotland.
Topics include diagnostic imaging innovations, what healthcare professionals need to know about occupational lung disease, recognising and managing tuberculosis and respiratory infections, critical care in respiratory medicine and the move towards personalised medicine and updates on targeted therapies.
The work of the multidisciplinary team is a theme running throughout the event, focusing on how respiratory physicians, surgeons, pharmacists, nurses and members of the wider clinical team can effectively and efficiently work together to provide better outcomes for patients.
The event is free to attend and comprises individual presentations, panel discussions and sponsored sessions delivered virtually live and on-demand, all tailored to provide maximum convenience and work around your busy schedule.
Pick and choose sessions most relevant to your clinical practice, specifically tailoring the day to your needs, and gain CPD hours from the comfort of your computer.
With a whole host of fascinating insights and inspiration for improving patient care, it’s not to be missed. Register now to join us on 1 May and on demand.
Don’t forget to check out Hospital Healthcare Europe’s respiratory Clinical Excellence section, brimming with content including interviews with prominent physicians to complement the event’s offering.
More Clinical Excellence events are in development for respiratory care and other specialities, which will be launching throughout 2024 – watch this space.
9th February 2024
The importance of integrated respiratory healthcare models that put patients at their centre has been outlined in a new joint position statement from the British Thoracic Society (BTS) and the Primary Care Respiratory Society (PCRS).
Aimed at all respiratory healthcare professionals across primary and secondary care, the position statement aims to improve health outcomes, tackle complex challenges and inequalities in the provision of healthcare, and prevent avoidable hospital admissions.
It highlights the common priorities shared by the BTS and PCRS and sets out a series of common goals to support the coordination of the multi-professional team and deliver high-quality, accessible care that considers physical and mental health, housing and social care.
The common goals focus on maintaining pathways of care that help to avoid unnecessary admission to hospital, advocating a whole-person approach to planning and delivering respiratory care, supporting improved outcomes and addressing health inequalities, and facilitating workforce recruitment, retention and capacity models.
These goals sit alongside each organisation’s own range of existing initiatives to support the provision of integrated care, they said.
The position statement also includes a five-part high level model for use by respiratory teams, detailing practical steps to help ensure the success of integrated respiratory healthcare: build relationships; identify funds; establish clear, identified goals; build the right team; and actively deliver the pathway.
Dr Paul Walker, chair of the BTS, said: ‘Integration of respiratory care is vital to optimally deliver health and social care in a system that is often disjointed and challenging for patients and carers to navigate. Not only is integrated respiratory care more efficient and productive it encourages sharing of skills, knowledge and insight.
‘This position statement encourages respiratory professionals and teams, across the healthcare landscape, to work better together to improve outcomes for patients.’
Daryl Freeman, chair of the PCRS’ Service Development Committee, associate clinical director primary care and GP in older people’s medicine in Norfolk, said: ‘It’s been an inspiration to be part of the BTS/PCRS joint working group delivering this statement.
‘I know that it will inspire clinicians, trusts and integrated care boards to design and deliver integrated care in their own regions and enable new implementers; giving them access to a document to which they can not only refer, but the support and experience of clinicians from BTS and PCRS who are either actively working in or developing integrated services.’
The BTS and PCRS represent and support all respiratory healthcare professionals working in the NHS across the UK.
The organisations hope that their collaboration on the position statement will help to ensure that tools, resources and education materials are shared widely across all members of the multi-professional respiratory team to the benefit of patients.