This website is intended for healthcare professionals only.
Take a look at a selection of our recent media coverage:
20th June 2024
Speaking at Hospital Healthcare Europe’s Clinical Excellence in Respiratory Care event, our panel of three experts considered the role of critical care in respiratory medicine. Dr Andrew Chadwick, Jane Scullion and Dr Phyllis Murphie PhD discussed how guidelines and best practice for treating respiratory patients in critical care have changed since the advent of non-invasive ventilation, as well as the lasting impact of the Covid-19 pandemic in this field.
With increasing use of non-invasive ventilation over the past 30 years, not to mention the wide-reaching impact of the Covid-19 pandemic, respiratory critical care has seen its fair share of change in best practice, which three Clinical Excellence event panellists know all too well.
Dr Andrew Chadwick is a respiratory and critical care consultant at Oxford University Hospital NHS Trust, where he is part of the nationally recognised special airways clinic. He reviews over 300 severe asthma, chronic cough and complex breathlessness patients each year and has a vast experience in critical care, as well as a self-professed love of non-invasive ventilation (NIV).
Independent consultant respiratory nurse Jane Scullion spent many years working at Glenfield Hospital, part of the University Hospitals of Leicester NHS Trust, across the TB service, asthma clinics, COPD services and interstitial lung disease clinics. In her early career, she was heavily involved in critical care and NIV on respiratory wards and, more recently, has worked in medical negligence and long Covid clinics.
Dr Phyllis Murphie PhD is an independent respiratory nurse consultant working in Dumfries and Galloway in Scotland. She specialises in sleep medicine and NIV, having introduced the NIV service into her hospital many years ago. In 2020, she led a respiratory nursing team through the Covid-19 pandemic and introduced changes to ensure the effective delivery of respiratory care during this challenging time.
Chaired by Garry McDonald, respiratory pharmacist at University Hospital Crosshouse in Scotland, the panel consider the trajectory of guidelines for managing respiratory failure, their take on ensuring patients respond well to NIV, and key learnings from the Covid-19 pandemic that are still in use at their hospitals today – including the somewhat divisive proning technique.
Dr Chadwick: It is fascinating how our mechanisms of treating respiratory failure have changed or haven’t changed and how we got here. If we wind our brains back, NIV starts getting developed in the late 80s and it starts to be used at home. It was late in the 90s when it exploded onto the scene in hospitals, with the work out of Leeds from Plant showing the staggering effect on COPD exacerbations. Number needed to treat: three to save a life. This is eye watering, it’s fabulous.
Then there is a real push in the early noughties of how far can we push these machines? What can we do? What works and what doesn’t? Then, you start getting a mixed signal in true acute respiratory failure. The narrative starts becoming: are we overusing this? Are we delaying intubation? Are we holding back, holding the patient in a period of risk?
There was a bit of controversy about it, and that built. Then, in 2020, a systematic review in JAMA pointed out that there are lots of small studies, put them all together, and what you start getting is a real signal of benefit of delaying intubating, a signal of benefit of saving life, and that helps us go forward.
Then Covid comes. A real challenge came out about are we sure we have got this right? And you had University College London at the start of this – we copied them in Oxford – saying, let’s hold people on continuous positive airway pressure (CPAP), let’s try and avoid incubating. Partly because we were worried we were running out of oxygen and I’m sure a lot of other places were too. And then the trial showed that this really does help to avoid intubating.
And now I think we’re left in a real limbo. I hope that gives a sense of where we are now, as I think where we are now is really interesting. If you were to go into the Berlin ARDS definition of guidelines, I think you’d find NIV spoken of in relatively negative terms in respiratory failure, apart from COPD, pulmonary oedema and cardiogenic shock. But I actually think in the real world, with really good data that I’ve hopefully just pinpointed, you find a much more balanced view and indeed a view that’s increasingly going: I wonder if we should hold, hold, hold off intubation.
If you look at the COPD literature, NIV to treat is unbelievably good compared to almost anything else in medicine. Initial trials using a pH of around 7.3 show significant improvements. I think in treating acute respiratory failure, increasingly, NIV can be a really good adjunct. Intubation is clearly the end of the road.
Ms Scullion: I remember the great big machines that you couldn’t carry around, trying to prop them up next to patients to ventilate them to start with. Now, we have lots of small portable devices, not just in a hospital setting, but people with longer-term conditions are also managing at home, especially with the LSAs [Lung Support Assistants] travelling with it.
It has been life transformative. Things do evolve. The nicest thing that came out of Covid was that respiratory proved that we could do the research, could look after the patients and could get results out of it that will alter as time goes on. We didn’t have enough critical care beds and the ordinary nurses with no respiratory background stepped up and did this, as did the doctors on the wards and pharmacists and physios. We ran almost mini high-dependency units wherever we could run them.
Dr Murphie: It’s remarkable, the evolution of the non-invasive ventilation story. From the days of my first job, I came to the consultant, who had two NIV devices in a cupboard and he didn’t know what to do with them. So, he said, ‘Phyllis, do you think you could arrange some training’, so it started from there.
When these devices were retired, we moved up to the next version, and then the next version, so the whole evolution of the devices that we used in secondary care, particularly during Covid, was a very steep learning curve for a lot of people because we had to do this outside of critical care in the wards.
Covid was the beginning of respiratory support units evolving. We spent hours and hours training all the staff to come in and be able to manage ward-based CPAP and NIV quite safely. For me, NIV really came of age at that point in time in terms of people’s understanding of it, when to use it and when not to use it.
Ms Scullion: It is really difficult when you have a patient in extremis, and you are going to put something onto the face. It is difficult when patients are really ill – you have to have a lot of time to get them to accept things.
I have often thought that as part of pulmonary rehab, especially with COPD patients, we should take these things in before people need them so they can see them and get used to it and feel what it is like because you can’t make a rational decision when you are extremely ill. We know some patients won’t tolerate it although a lot do. It can be uncomfortable, it dries your mucosa and there are all the other side effects. So, it is time and patience.
Dr Murphie: Something that is really important is knowing how to mask fit properly. Being able to make sure they have got the right size mask on, because then you start getting pressure sores and things on the nose. Fitting the masks and making sure that people know how to fit them properly and not do harm is a really important skill to learn as well.
It takes a certain level of skill to acclimatise your patient onto the therapy. You have to be patient, work out the fears and talk them through it. Sometimes, you have to start with the sub-optimal measures to get them comfortable and confident enough to wear the mask. You give them reassurance that this is something that works really well; it could shorten the length of time they are in the hospital and make the other therapies work better as well.
Dr Chadwick: This is one of those times when you really need to add in all your confidence, and you need to get the patient to buy into it. Don’t underestimate the power of reassurance and the power we have as healthcare professionals to do that.
So, coming in, being reassuring and then asking for one hour of NIV and really trying it. Then you can judge the blood gas, and you can go back to them and their family, and say, ‘look, we really tried, but we’re not winning with this, so let’s not’.
Or you can say, ‘Actually, look, we have really made a big progress; our pH has jumped from 7.1 to 7.15, so that’s a huge difference’. Then suddenly, you are in a new conversation saying, ‘well, actually, I’ve got physiological proof that this works for you, so work with me. This is going to be brilliant’.
Dr Murphie: Having outreach teams is really important. Making sure that we can talk to the teams in, say, the combined assessment unit. They want to see patients early. If they are starting to struggle, and you can see that their blood gases are going off, then we want to know early on.
Getting in early and trying to work with the patient to reassure them that there is something worth trying to see if they can feel better. They can turn a clock back very quickly and start to see improvements if it is applied early enough and not too late.
Dr Chadwick: You just jumped onto one of my pet peeves. Number needed to treat is unbelievably good – better than almost everything else in medicine. Increasingly, what you are seeing is drift in all of us, in every clinical practice, we are just holding it back later when the patient is sicker. Early is better. You get in there early and stop the hypercapnia, if that’s what you’re doing with NIV. It’s much easier than coming in when they are really down the line. It’s a real pet peeve of mine: what are we doing holding back? There is a kind of odd culture of holding NIV back.
Ms Scullion: The acceptance of patients is better if they are not confused and not fighting it and not agitated and not desperately ill. That has to be the best option to do it as soon as possible.
We want to do the best for the patients in front of us, and sometimes NIV is the best treatment that you can give, and it stops a lot of other things. Our patients nowadays do get fully ventilated and do get off ventilators, but not in great numbers.
A lot of them do poorly, and it is not a terribly nice prognosis at the end for the family to cope with. So, NIV, for me, is a nicer option because the patient is still in the room with their loved ones.
Ms Scullion: I was so proud of the respiratory community during Covid because we had to get on with it. A lot of the decisions were made by clinicians. We did for patients what we could and everybody – across the board, pharmacists, physios, put their shoulder to the wheel and did it. Even in patients when we were proning, and things like that, you know there were 10 or 12 people proning a patient.
I mean, proning was something where, if you can say, good came out of Covid because it worked. It’s probably quite an old technique. I’ve seen the pictures of the old machines where people were turned in the machines and had a mirror so they could see up or a mirror that could see down. So, it has been around for a long time and often, just because it’s old, doesn’t mean it’s not good.
Dr Chadwick: It is perceived as an old trial. I was working out in Paris briefly on a long placement and they, honestly, were flabbergasted pre-Covid that we don’t just prone everybody. And in England, the problem is – and this is common across units and there’s no judgement because these are world-class British units – but we would always say things like, ‘oh, it’s not safe, you might dislodge, you might do something’. And you’re absolutely right, Covid put that all to the wind.
Work done by people like UCL really nudged the needle back to say, ‘come on now, prone them, it really works. It buys you space to ventilate them kindly and keep within those safe parameters’. So, you’re absolutely right, Jane, it’s another fabulous example of where real positives came out of Covid and essentially just reset that needle and how we treat acute respiratory failure.
Dr Murphie: We had the Army logistics teams come in and they basically organised and changed the flow of the hospital. Dumfries and Galloway is a brand new hospital with all single rooms, which was fantastic. The air changes in each single room six times per hour, so we actually really didn’t have a huge amount of in hospital transmission.
We moved the respiratory ward right along to the other end of the hospital so we were very close to the combined assessment unit. When patients were being moved, there was a green flow and we had a red flow. The green flow was the clean way to go and the red for the contaminated way. So, that really changed the way in which we actually managed patients in the hospital and we had a command control structure that did work really, really well in that environment and it served a purpose at that particular time and helped us to think about how we carry on and give safe care in the really, really difficult place that we were all working.
And that brings me to the point about MDT working. It was fantastic. Every morning, at nine o’clock on the ward, we would have a huddle and every single discipline was there to actually be involved in everything that we needed to do that day with the patients.
For me, the shining stars were the physios and the occupational therapists (OTs). They were so good at trying to get people on their feet. Anybody who had been in critical care and had been ventilated, they’d lost so much of their muscle tone, health, you name it. And the physios and the OTs got them back on their feet and got them home again and it really did shine a light on how great our MDT colleagues are.
Dr Chadwick: We had loads of colleagues, like our vascular surgeons, who came and said, ‘we’re here to help’. The way our respiratory MDT started setting up was that we gave them a physio to lead them as a proning team. There’s this wonderful image of Annika who’s an amazing physio and quite a petite lady, and these six quite bulky vascular surgeons turning this patient. But they learned very quickly that the rules were you just do what Anika says to the letter. It was serious because you’re turning someone on a ventilator – you can really muck it up – but it was really wonderful to watch.
Exactly as you describe, Phyllis, it was fabulous MDT working. And that’s actually stayed with us in Oxford: to this day: we do a lot more proning and our physios still run our proning teams, not our doctors. We’ve decided that they do a better job, and therefore that’s very much left with them. Whoever’s there doing the proning, be that a consultant or whoever it is, that doesn’t matter as in that moment, the physio is in charge. We listen to them, we do what we’re told and we prone very safely.
I think acute respiratory failure is just a lovely example of a bit of medicine where the MDT does make it all work. If you took any one cog away, all of it falls away.
22nd May 2024
People with multiple sclerosis (MS) have a much higher risk of being hospitalised and dying from Covid-19 than the general population, despite being more likely to be vaccinated, UK research suggests.
The analysis was done on data from the INvestigation oF cOvid-19 Risk among iMmunocompromised populations (INFORM) study which includes information on 12 million people aged 12 years and older in England.
Previous research from the study which was set up to investigate the impact of Covid-19 among people who are immunocompromised has shown substantially higher risk of severe outcomes in this population.
But until now people with MS had not been included in that category and not looked at in detail, the researchers said.
A comparison of Covid-19 infections in vaccinated people with MS and the general population across 2022 showed a seven-times greater risk of Covid-19 hospitalisation and four-fold increased risk of dying in those with the condition.
Overall, the researchers looked at health records from 16,350 people with MS and found three-quarters had received at least three doses of Covid-19 vaccine compared with half of those in the general population.
Among individuals with MS, there were 215 Covid-19 hospitalisations and 25 Covid-19 deaths, equating to incidence rates of 1.28 and 0.14 per 100 person-years, respectively.
This compared with rates of 0.24 and 0.06 per 100 person-years for hospitalisations and deaths in the general population.
Presenting at the European Society of Clinical Microbiology and Infectious Diseases congress, ESCMID Global, in Barcelona, the researchers will say this highlights the ‘urgent need’ for preventive measures for people with MS who are inadequately protected by vaccination alone.
However, the team did not look at what disease-modifying therapies patients might have been taking and the impact on risk.
Study lead Professor Jennifer Quint, professor of respiratory epidemiology in the School of Public Health at Imperial College London, said: ‘Having multiple sclerosis in itself doesn’t increase the risk of getting Covid-19, rather it’s the taking of immune-modifying medicine that can reduce the effectiveness of vaccines by preventing the immune system from mounting a robust protective response.‘
She added: ‘Some MS-specific factors, such as having underlying conditions or higher levels of disability can contribute to poor outcomes.
‘As a result, even after repeated doses of Covid-19 vaccines, some individuals with MS remain at high risk of serious outcomes.’
Professor Quint said she hoped the findings would raise awareness that the threat of Covid-19 is still very real for many individuals and that booster vaccines are inadequate to fully protect them.
‘With new variants constantly emerging, people living with MS should be considered an important high-risk group for Covid-19 hospitalisation and death for which additional preventive measures and multi-layered public health protections are urgently needed,’ she concluded.
A version of this article was originally published by our sister publication Pulse.
27th February 2024
Waking critically ill Covid-19 patients for rehabilitation during prolonged extracorporeal membrane oxygenation (ECMO) therapy can improve their prognosis, a new study has found.
Seriously ill Covid-19 patients usually needed a longer period of extracorporeal support than other acute respiratory distress syndrome (ARDS) patients, researchers explained in the journal Annals of the American Thoracic Society.
To understand the factors influencing prognosis and the benefits of a wake-up strategy, researchers conducted a retrospective observational study of 120 patients with Covid-19-related ARDS who required extracorporeal support for more than 28 days from four European high-volume ECMO centres.
Analysing patient outcomes, researchers found 64 people (53.3%) survived decannulation, 62 (51.7%) were alive at hospital discharge and 61 (50.8%) were alive at six-months follow-up.
In a multivariate analysis, younger age was found to be a predictor of hospital survival together with an awake ECMO strategy – defined as the patient being awake, cooperative, and performing rehabilitation and physiotherapy with or without invasive mechanical ventilation at any time during the extracorporeal support.
‘Sedation withdrawal and active rehabilitation are feasible in patients with extracorporeal respiratory support, given that ECMO offers an efficient way of maintaining an adequate gas exchange with the potential of titrating the support according to the patient’s oxygen consumption,’ the researchers wrote.
More than 75% of the cohort of patients receiving the awake ECMO strategy were alive at six months, the researchers said, compared with 41.4% of the most inactive cases.
Patients who were woken during ECMO also required less rehabilitation after discharge, with 68.2% of them requiring discharge to a rehabilitation centre compared with 80.6% of inactive patients.
Senior study author Dr Jordi Riera, director of the adult ECMO program at Vall d’Hebron University Hospital, Spain, and principal investigator of the hospital’s research group on Shock, Organ Dysfunction and Resuscitation, said there were many advantages to the wake-up strategy, including interaction with relatives which offered cognitive and emotional benefits.
‘In addition, if healthcare professionals can communicate with patients, a more accurate diagnosis can be made based on the symptoms they are explaining and, therefore, therapy can be better adjusted,’ said Dr Riera.
Waking patients also minimised some complications, he said, including fewer infections, as patients were able to cough, and less lung damage associated with ventilation.
Although this study was focused on patients with Covid-19, the researchers suggested these results could be generalisable to patients with respiratory dysfunction from other causes.
They also emphasised that despite requiring ECMO for one month, it was possible for these critically ill Covid-19 patients to make a full recovery without the need for oxygen therapy.
At six months, 51 (42.5%) patients were at home, 42 (84.3%) of them without oxygen therapy, the researchers highlighted.
However, longer duration of ECMO support was correlated with need for oxygen therapy at six months.
A cut-off point of 47 ECMO days had a 100% sensitivity and 60% specificity for oxygen therapy at six months, with 100% specificity being found in 97 days.
‘In our view, this cut-off point should be used as a reminder of the possibility of non-recovery and may be used as a trigger for initial contact with an experienced [lung] transplant team for assessment and advice,’ the study authors wrote.
‘However, it must be noted that oxygen therapy on itself is not an indication for lung transplant and that complete recovery has been observed in patients supported with ECMO up to 97 days in this very same cohort.’
16th January 2024
People not having all their recommended doses of the Covid-19 vaccine led to thousands of unnecessary deaths and severe outcomes, researchers have found.
Over 7,000 hospitalisations and deaths might have been avoided in summer 2022 if there had been ‘full’ Covid-19 vaccination coverage across the UK, according to a new study published in The Lancet,
Using health record data from participants over four years old, researchers found that between a third and a half of the populations of the four UK nations had not received the recommended number of Covid-19 vaccinations by summer 2022.
Over the period between the start of June and the end of September that year, they used a ‘counterfactual scenario’ in which everyone in the UK was fully vaccinated in order to estimate the potential reduction in ‘severe Covid-19 outcomes’.
The study concluded that there would have been 7,180 fewer hospitalisations or deaths across all age groups, down from the 40,393 that actually took place, indicating that ‘higher vaccination coverage would have been associated with considerable reduction in severe Covid-19 outcomes’.
Among the undervaccinated, the researchers estimated there would have been a 50% reduction in these events.
They also found that younger, more deprived or non-white people, or those with a lower number of comorbidities, were less likely to be fully vaccinated.
The study defined ‘undervaccination’ as having received fewer than the recommended number of vaccine doses.
Rates of ‘undervaccination’ against Covid-19 across the UK as of 1 June 2022 were:
‘The effect of being undervaccinated on severe Covid-19 outcomes was notably larger than the effect of ethnicity or socioeconomic status,’ the researchers concluded.
They also said health policy and public health interventions need to be formulated with the aim of improving coverage among the ‘undervaccinated’ demographics.
‘This could, for example, include the need to tackle vaccine misinformation in a more direct fashion, and to continue to diversify the use of champions to support public messaging and the range of community-based centres offering vaccinations,’ the study said.
Dr Andrew Freedman, consultant in infectious diseases and vaccine clinical trials at Cardiff University School of Medicine, said the study ‘clearly demonstrates how effective Covid vaccines were at reducing the risk of severe outcomes’ from the disease during summer 2022.
He said: ‘As the authors point out, these findings will help identify which groups should in future be specifically targeted to maximise uptake of vaccines for Covid and other infections. This will be particularly crucial in the event of another pandemic.’
Dr Freedman also noted that the study’s inclusion of data from the whole UK population – the first of its kind – is a ‘remarkable achievement’.
A version of this article was originally published by our sister publication Pulse.
12th January 2024
Around 1.4 million more people in the UK will be eligible for antiviral treatment nirmatrelvir plus ritonavir (brand name Paxlovid) if they test positive for Covid-19 after final draft guidance from the National Institute for Health and Care Excellence widens access.
It follows a partial review of the evidence that identified additional groups of people who are at increased risk of severe Covid-19.
Under the recommendations, nirmatrelvir plus ritonavir will be available after a positive Covid-19 test to people aged 85 and over, as well as people who are resident in a care home or are already hospitalised and are aged 70 years and over, have a body mass index greater than 35 kg/m2 or have diabetes or heart failure.
People with end-stage heart failure who have a long-term ventricular assistance device and those on the organ transplant waiting list will also be eligible.
The eligibility is in addition to the 3.9 million people who were already identified as being as increased risk of progression to severe illness if they were infected with Covid-19, NICE said.
Those who are eligible can get free lateral flow tests from participating pharmacies and should take a test as soon as they have symptoms, even if mild, NICE reiterated before calling their GP, NHS 111 or hospital specialist if they test positive.
Helen Knight, director of medicines evaluation said: ‘Our review of the evidence on the use of Paxlovid has found it offers value for money for a wider group of patients.
‘This is good news for people who may contract Covid-19 in the coming months and will help alleviate pressure on the health service.‘
She added: ‘Although we are no longer in a pandemic, Covid-19 is still circulating and we are pleased that more people at risk of severe disease can benefit from Paxlovid.’
Nirmatrelvir plus ritonaviris is an antiviral medicine, given as two separate tablets to people within five days of Covid-19 symptom onset.
Nirmatrelvir stops the virus from growing and spreading, and ritonavir helps nirmatrelvir from being broken down in the body while it is working.
UK public health officials have reported rising levels of winter bugs in the past week with rates of flu, Covid-19 and norovirus continuing to rise.
The latest figures also show a rise in Covid-19 hospitalisations in the last week of 2023 as well as patients admitted to ICU with complications of the virus.
8th November 2023
There was a more rapid cognitive decline in people aged over-50 during the pandemic, according to recent research, highlighting the need for public health measures to protect against dementia risk.
Covid-19 infection was found to be a risk factor, but the general more rapid decline in brain health was apparent even if people had not had the virus, the researchers found.
Published in the journal The Lancet Healthy Longevity, the online PROTECT study found a 50% change in the rate of cognitive decline in the first year of the pandemic among the 3,000 participants.
The analysis of tests of short-term memory and complex tasks also found that the rate of cognitive decline was higher in those who already had mild cognitive decline before the pandemic.
It appears to have been exacerbated by a number of factors among the 50-to-90-year-olds taking part, including an increase in loneliness and depression, a decrease in exercise and higher alcohol consumption.
In the second year of the pandemic, reduced exercise continued to affect executive function and associations were sustained between worsening working memory and increased alcohol use. This suggested a sustained impact after the initial 12-month period of lockdowns, the team from the University of Exeter and King’s College London (KCL) said.
The sustained drop in cognition highlights the need for public health interventions to mitigate the risk of dementia – particularly in people with mild cognitive impairment where diagnosis of dementia within five years is a substantial risk, they concluded.
Professor Anne Corbett, professor of dementia research and the PROTECT study lead at the University of Exeter, said: ‘Our findings suggest that lockdowns and other restrictions we experienced during the pandemic have had a real lasting impact on brain health in people aged 50 or over, even after the lockdowns ended.
‘This raises the important question of whether people are at a potentially higher risk of cognitive decline which can lead to dementia.
‘It is now more important than ever to make sure we are supporting people with early cognitive decline, especially because there are things they can do to reduce their risk of dementia later on.’
She added that the findings highlight the need for policymakers to consider the wider health impacts of restrictions like lockdowns when planning future pandemic responses.
Professor Dar Aarsland, professor of old age psychiatry at KCL, said the research adds to the knowledge of the long-standing health consequences of Covid-19, particularly for the most vulnerable people such as older people with mild memory problems.
He said: ‘We know a great deal of the risks for further decline, and now can add Covid-19 to this list. On the positive note, there is evidence that lifestyle changes and improved health management can positively influence mental functioning. The current study underlines the importance of careful monitoring of people at risk during major events such as the pandemic.’
A version of this article was first published by our sister publication Pulse.
6th November 2023
Tens of thousands of people are suffering with long Covid symptoms more than a year after infection, say researchers who carried out the UKs largest study of the impact of the virus.
A survey of a quarter of a million people taking part in the REACT study found that while most people recover within 12 weeks, 7.5% of people had persistent symptoms for more three months and 5% reported symptoms lasting more than a year.
But people infected in the Omicron wave of the pandemic were 88% less likely to experience symptoms longer than four weeks after infection, compared to earlier wave, the study found, which could be due to immunity building up in the population and vaccination.
The new analysis, published in the journal Nature Communications, also highlighted how persistent symptoms of Covid-19 were related to worse mental health and quality of life.
Being female, already having more than one comorbidity, being from a deprived area, and being infected with an original strain of Covid-19 were all related with higher risk of symptoms lasting more than 12 weeks and longer recovery time in those with persistent symptoms, the researchers said.
The survey results – which were collected in towards the end of 2022 – show a snapshot of the continued impact of Covid-19 in the UK, the Imperial College London team noted.
Mild fatigue, difficulty thinking or concentrating and joint pains were the most common ongoing symptoms. But others reported loss or change of sense of smell or taste, shortness of breath, severe fatigue, chest tightness or pain, and poor memory.
Almost a third of people reporting symptoms at 12 weeks recovered within a year, the researchers found.
Professor Paul Elliott, chair of epidemiology and public health medicine at Imperial College London, said: ‘We find that the variant of SARS-CoV-2 people are infected with, the initial severity of their symptoms, and whether they have pre-existing health conditions all have an impact on whether they will develop lasting symptoms.’
Study lead Dr Christina Atchison, principal clinical academic fellow within the School of Public Health at Imperial College London, said while the landscape has changed considerably since the early peak of the Covid-19 pandemic, this analysis shows that a proportion of adults are still experiencing lasting symptoms.
‘Importantly, we find that compared to wild type virus, those infected when Omicron was dominant were far less likely to report symptoms lasting beyond 12 weeks,‘ she said. ‘This may reflect the changing levels of immunity in the population from previous exposure to the virus and vaccination.’
The team is now doing detailed interviews with some of those affected with ongoing symptoms to further understand the variation in people’s experiences and the impact on their everyday lives as well as the broader longer-term impact of the pandemic on health and wellbeing of those who took part in REACT.
This article was originally published by our sister publication Pulse.
27th September 2023
The benefits of ensitrelvir use in patients with symptoms of long Covid and as a second-line treatment option for those hospitalised with Covid-19, have been presented at the recent European Scientific Working Group on Influenza and other Acute Respiratory Viruses’ (ESWI) Influenza Conference.
Ensitrelvir is an oral antiviral drug for Covid-19 manufactured by Shionogi, which is currently approved under the emergency regulatory approval system in Japan. The drug inhibits 3CL protease, an enzyme that is essential for the replication of the virus.
The first study presented at the ESWI Influenza Conference was an exploratory analysis of the Phase 3 part of the pivotal SCORPIO-SR trial. Conducted in Japan, South Korea and Vietnam, the researchers found that ensitrelvir may reduce the risk of a number of persistent and new late-onset symptoms associated with long Covid over one year.
Long Covid was analysed based on a patient-reported questionnaire at three, six and 12 months from the first date of treatment. The researchers defined long Covid by patient reports of ‘not having returned to pre-Covid health’ and having at least one (mild or more severe) symptom out of 27 possible symptoms.
The use of ensitrelvir at doses of 125 mg and 250 mg, showed a numerical relative risk reduction of 25% and 26% respectively when compared to the placebo for 27 long Covid symptoms at one year. A similar trend in risk reduction was observed at three and six months.
Furthermore, in subgroup analyses, there was a greater risk reduction in patients with a body mass index of ≥25 kg/m2 as well as in those with a median or higher symptom score at the start of treatment.
These results suggest that early treatment of Covid-19 with ensitrelvir could reduce the risk of a number of persistent and new late-onset symptoms associated with long Covid.
Dr Andreas Karas, vice president, medical affairs, Shionogi Europe, said: ‘As Covid-19 remains endemic, persisting symptoms continue to adversely affect millions of people worldwide. We’re pursuing further research to substantiate the potential of ensitrelvir as a treatment option for acute Covid-19 infection and whether this can prevent the long-term effects the virus can cause.
‘We look forward to sharing the results at ESWI and to hearing from the community as we advance our global ensitrelvir clinical development program.‘
In the second study presented at the ESWI Influenza Conference, researchers suggested that for hospitalised patients with Covid-19 and significant co-morbidities who failed to respond to remdesivir as the first-line treatment, ensitrelvir could serve as an alternative treatment.
The small, real-world study from Japan, conducted at the Rinku General Medical Centre, included 21 high-risk hospitalised patients with Covid-19 infections who failed to respond to a three-day course of remdesivir.
These patients, who had either a mild or moderate severity infection and of whom a third (33.3%) had co-morbidities, then received five days of treatment with ensitrelvir 125 mg.
After five days of treatment, all 21 patients showed clinical improvement. Furthermore, no deaths or drug-related adverse events were observed at day 28, but one person later died in the hospital at day 59.
Masaya Yamato, an investigator on these trials and director of the Infectious Diseases Center at Rinku General Medical Center in Osaka, Japan, said: ‘While certain treatments are available for high-risk people with Covid-19 to reduce the risk of becoming seriously ill, additional treatment options are needed. These results indicate the potential for ensitrelvir to help address these needs.‘
Ensitrelvir remains an investigational drug outside of Japan. In April 2023, the FDA in the US recently granted fast track designation to the drug for Covid-19. This process is designed to facilitate the development and expedite the review of potential new therapies that treat serious conditions and fulfil an unmet medical need.
7th July 2023
Patients with higher prior antibiotic use are more likely to experience severe Covid-19 outcomes after infection, including hospitalisation and death, a new study led by the University of Manchester has shown.
When infected with Covid-19, the chances of dying from complications due to Covid-19 is 1.34 times higher in patients with the most exposure to antibiotics than those without antibiotic exposure.
The research, published in The Lancet‘s eClinicalMedicine, is the first to explore how the severity of Covid-19 is affected by prior antibiotic use.
The UK team of scientists behind the work say the findings should act as a warning against the overuse of antibiotics.
Using the NHS OpenSAFELY platform, which enabled the researchers to look at electronic health records across primary and secondary care, the team examined data from 670,000 patients infected with Covid-19 between February 2020 and December 2021. The patients were reviewed for Covid-19 outcomes and divided into five groups based on the frequency with which they had taken antibiotics three years prior to infection.
Each group was further split based on the number of different antibiotics a patient used, giving the researchers an understanding of how the frequency and diversity of antibiotic use affect the body’s response to a Covid-19 infection. Of the sample, 98,420 patients were admitted to hospitals, 22,660 died, and 55 unique antibiotics were prescribed.
Patients with more frequent antibiotic exposure in the past three years were more likely to experience severe Covid-19 outcomes, including admission to hospital and death within 30 days.
The odds of being hospitalised due to a Covid-19 infection were 1.8 times higher for those with the highest history of antibiotic use and the most diversity of antibiotic use. Using a range of antibiotics was more likely to be associated with Covid-19 hospital admission, and using a larger range of antibiotic types was associated with more severe consequences of Covid-19 upon hospital admission.
Professor Tjeerd van Staa, a principal investigator from the University of Manchester, said: ‘Our study has provided evidence that patients with high prior antibiotic use were more likely to experience severe Covid-19 outcomes. In addition, we also found an association between the number of different prior antibiotic types and Covid-19 related hospital admission.’
The researchers suggest this may be because frequent antibiotic use can increase the likelihood of patients being infected with viruses or bacteria, leading to an increased susceptibility to adverse consequences of infection.
Professor Van Staa added: ‘The literature shows that antibiotic treatment might alter gut microbiota, which can impact metabolic and immune function. While in most situations gut microbiota will recover after stopping an antibiotic course, frequent antibiotic use may affect the resilience of gut microbiomes more seriously.’
The researchers suggest that antibiotic guidelines should outline the possible adverse consequences of the overuse of antibiotics, and personalised patient leaflets could highlight these risks and the risks of the patient’s bacteria developing resistance to antibiotics.
Co-principal investigator Dr Victoria Palin from the University of Manchester said: ‘Common infection guidelines in England, as developed by the National Institute for Health and Care Excellence, focus on the treatment of the first infection episode. They do not provide guidance around repeated antibiotic use and a patient’s risk of developing resistance.’
She added: ‘There needs to be more awareness of the impact of long-term antibiotic exposure and its adverse outcomes. We would discourage regular and indiscriminate prescribing of these drugs for self-limiting infections.’
This article was originally published by our sister publication Nursing in Practice.
4th July 2023
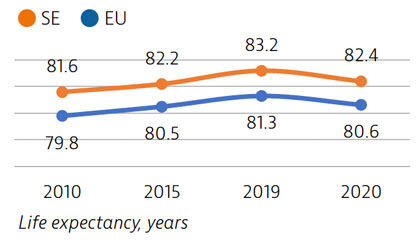
Life expectancy in Sweden is among the highest in the EU, although it declined by nearly one year in 2020 as a result of the Covid-19 pandemic. The healthcare system generally performs well in providing good access to high-quality care.
However, challenges persist in providing equal access to care for the population living in different regions, ensuring timely access to care, achieving greater care coordination for people with chronic diseases and improving the quality of long-term care.
Life expectancy at birth was 82.4 years in 2020 – almost two years above the EU average – but it fell by almost one year in 2020 because of the high number of deaths from Covid-19. More than two thirds of Covid-19 deaths were among people aged 80 and over.
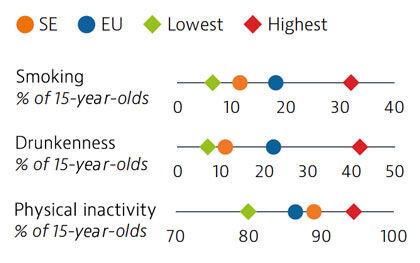
Smoking rates among adults in Sweden are among the lowest in EU countries, but use of other tobacco products such as snuff is common. Overall alcohol consumption per adult has decreased over the past decade and is much lower than the EU average. Adolescents in Sweden also report low rates of smoking and excessive alcohol intake, but high rates of physical inactivity.
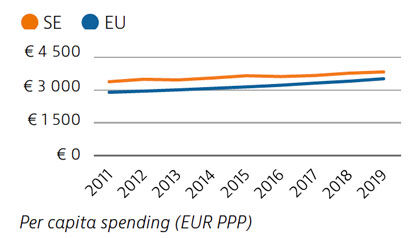
Health spending per capita in Sweden was the fourth highest in the EU in 2019, and the third highest in terms of health spending as a share of GDP. Most health spending is publicly funded (85%). The growth rate in health spending was relatively modest in the years prior to the pandemic, but the government increased spending on health in 2020 and 2021 in response to Covid-19.
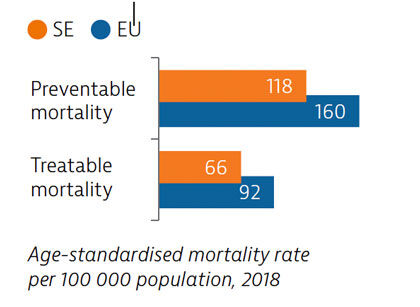
Sweden had low rates of mortality from preventable and treatable causes in 2018, pointing to a generally effective public health and healthcare system under normal circumstances.
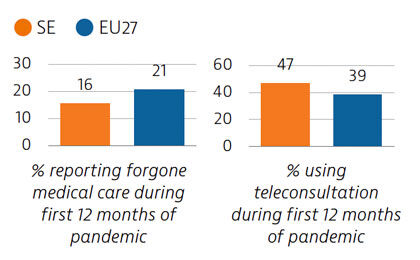
During the first year following the pandemic, one in six people in Sweden reported some unmet needs for medical care, which is slower than the EU average. The use of teleconsultations increased quickly in Sweden during the pandemic to maintain access to care.
Sweden tried to balance protection of people’s health and protection of economic and social activities in managing the Covid-19 crisis. While fewer restrictions were imposed, particularly during the first wave, the death toll was high compared with other Nordic countries. By end of August 2021, 58% of the population had received two doses or the equivalent – slightly more than the EU average.
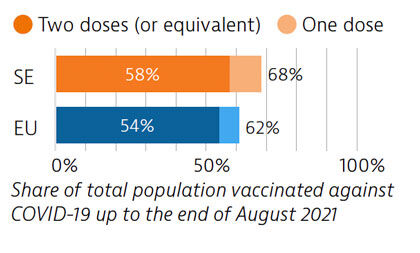
OECD/European Observatory on Health Systems and Policies (2021), Sweden: Country Health Profile 2021, State of Health in the EU, OECD Publishing, Paris/European Observatory on Health Systems and Policies, Brussels.