This website is intended for healthcare professionals only.
Take a look at a selection of our recent media coverage:
5th February 2024
Both the global cancer burden and inequity in cancer and palliative care services are growing across the world, according to new figures released by the World Health Organization (WHO).
Published to coincide with World Cancer Day (4 February 2024), the survey undertaken by the WHO’s cancer agency, the International Agency for Research on Cancer (IARC), shows a growing need for more cancer-related health services worldwide.
Over 35 million new cancer cases are predicted in 2050, a 77% increase from the estimated 20 million cases in 2022, the IARC said.
This ’rapidly growing global cancer burden’ reflects population ageing and growth, as well as changes to people’s exposure to risk factors such as tobacco, alcohol, obesity and air pollution, it added.
The figures show that 10 types of cancer collectively comprise around two-thirds of new cases and deaths globally. Lung cancer is the most commonly occurring cancer worldwide, followed by female breast cancer and colorectal cancer.
In 2022, there were an estimated 20 million new cancer cases and 9.7 million deaths, with lung cancer accounting for 2.5 million or 12.4% of the total new cases.
Female breast cancer ranked second with 11.6% of new cases, followed by colorectal cancer, which accounted for 9.6% of new cases. Prostate cancer and stomach cancers were the next two most common, respectively.
Lung cancer was also the leading cause of cancer death, accounting for nearly a fifth of the total cancer deaths, followed by colorectal cancer 9.3% of deaths and liver cancer 7.8% of deaths.
Differences were seen between sexes, with breast cancer being the most commonly diagnosed cancer and leading cause of cancer death amongst women, whereas it was lung cancer for men.
Prostate and colorectal cancers were found to be the second and third most commonly occurring cancers for men, with liver and colorectal cancers second and third most common causes of cancer death.
For women, lung and colorectal cancer were second and third for both the number of new cases and of deaths.
Lung cancer’s re-emergence as the most common cancer is likely related to persistent tobacco use in Asia, the IARC said.
The figures also show that a majority of countries do not adequately finance priority cancer and palliative care services.
Only 39% of participating countries covered the basics of cancer management as part of their funded core health services for all citizens, and only 28% of participating countries additionally covered care for people who require palliative care.
In areas where patients are underserved in relation to cancer treatments, rates of cancer are higher, highlighting a growing inequity in cancer services worldwide.
Dr Panagiota Mitrou, director of research, policy and innovation at the World Cancer Research Fund, stated that the UK Government needs to prioritise cancer care in light of the increasing number of global cases.
She said: ‘These new estimates show the increased burden that cancer will have in the years to come. UK Governments’ failure to prioritise prevention and address key cancer risk factors like smoking, unhealthy diets, obesity, alcohol and physical inactivity has, in part, widened health inequalities. We know around 40% of cancer cases could be prevented.’
She added: ‘Now is the time to turn the tide by implementing policies that enable people to live healthier lives by reducing their exposure to risk factors and prioritising a national cancer plan which includes better screening and early detection.’
The WHO figures come as NHS England announces the launch of a national gene testing programme to identify cancer risk for people with the BRCA gene.
The BRCA refers to two genes, BRCA1 and BRCA2, which repair DNA damage and help to protect against cancer. If one of the genes is faulty, this can increase a person’s chance of getting cancer significantly.
People with Jewish ancestry are around six times more likely to carry such genetic faults than the rest of the population and are therefore at increased risk of developing some cancers.
Through genetic testing, the NHS plans to identify people carrying faults in the BRCA gene to ensure those affected have access to early surveillance and prevention services.
People with at least one Jewish grandparent can register for a saliva test kit, and following the success of the pilot programme, it is expected that the national roll-out will see around 30,000 people tested over the next two years.
Commenting on the programme, Peter Johnson, national clinical director for cancer at NHS England, said: ‘BRCA testing for the people most at risk has the potential to save lives by allowing them to take steps to reduce the chance of cancers developing or making sure that any cancer can be detected as early as possible, with those at increased risk able to take advantage of surveillance and prevention programmes with their health teams.’
A version of this article was originally published by our sister publication Nursing in Practice.
18th January 2024
Subcutaneous atezolizumab (brand name Tecentriq SC) has been granted marketing authorisation by the European Commission (EC) for multiple cancer types, its manufacturer Roche has announced.
It is the first PD-(L)1 cancer immunotherapy for subcutaneous (SC) injection available in the European Union and offers the potential for a faster and more convenient alternative to intravenous (IV) infusion of atezolizumab.
The SC injection is administered under the skin and takes approximately seven minutes to complete, with most injections taking between four and eight minutes. This is 75% quicker than the 30-60 minute duration of the IV infusion.
The EC approval covers all indications in which the IV form of atezolizumab has previously been approved, including certain types of lung, liver, bladder and breast cancers.
In August 2023, the NHS in England became the first health system in the world to roll out SC atezolizumab after its approval by the Medicines and Healthcare products Regulatory Agency (MHRA).
The EC and MHRA approvals were based on pivotal data from the Phase IB/III IMscin001 study, which showed comparable levels of atezolizumab in the blood, when administered subcutaneously, and a safety and efficacy profile consistent with the IV formulation.
Some 90% of healthcare professionals who were surveyed as part of the study agreed that the SC formulation is easy to administer and 75% said it could save time for healthcare teams compared with the IV formulation.
While the IMscin001 trial was conducted within the hospital setting, SC atezolizumab may be administered by a healthcare professional outside of the hospital in a community care setting or at a patient’s home, depending on national regulations and health systems.
Commenting on the approval, Dr Enriqueta Felip, head of the Thoracic Cancer Unit of Vall d’Hebron Hospital in Barcelona, Spain, said: ‘Ensuring the best possible quality of life is crucial for people living with cancer.
‘The availability of a subcutaneous cancer immunotherapy option that can minimise the time receiving treatment and even allow for treatment outside of a hospital will undoubtedly make a significant difference to patients and their loved ones.’
Levi Garraway, Roche’s chief medical officer and head of global product development, added: ‘Giving Tecentriq subcutaneously provides more flexibility to patients, while also helping to free up resources in constrained healthcare systems.’
Roche is working with national health systems in Europe to ensure patients can access SC atezolizumab as quickly as possible, and the company is also in discussion with several providers in Europe to include the drug in cancer homecare initiatives where possible.
19th December 2023
A new app designed to reduce isolation and mental burden among young cancer patients is to undergo a national rollout across Denmark in 2024 after a successful pilot.
The ‘Kræftværket‘ (The Cancer Forge) app provides teenagers and young adults in cancer treatment with a comprehensive suite of features including symptom tracking, access to health information and a supportive online community with whom to share treatment experiences.
The app aims to alleviate loneliness and enhance young people‘s mechanisms for coping with their disease and was developed in collaboration with young cancer patients and healthcare professionals.
It is the first digital health solution of its kind in Europe targeted at young cancer patients for national health system implementation.
Professor Helle Pappot, professor in clinical oncology at University of Copenhagen and clinical professor at Rigshospitalet, University Hospital of Copenhagen, Denmark, was instrumental in the app‘s development.
She said: ‘The app has proven to be a good tool for creating an overview of and mastering one‘s illness, that both the healthcare system and the patients can benefit from. Our research shows that young people find the app supportive and meaningful.
‘The solution has been a crucial tool in empowering patients to manage their disease, and through our clinical research we have been able to document the positive impact on a patient’s quality of life.‘
A pilot study led by the University of Copenhagen, which was published in the journal JMIR Mhealth Uhealth in 2019, investigated the feasibility of a smartphone app among adolescent and young adult patients with cancer in active treatment and post-treatment.
A total of 20 participants, 10 in active treatment and 10 in post-treatment, were recruited at Rigshospitalet and asked to use the Kræftværket app as they deemed appropriate over a six-week period.
The participants were asked to complete the 30-item European Organization for Research and Treatment of Cancer Quality of Life (QoL) Questionnaire before and after the six-week period.
The post-treatment group experienced a significant increase in overall QoL after the six-week period (global QoL: baseline 62.5, SD 22.3; after six weeks 80.8, SD 9.7; P=.04).
For the group in active treatment, the QoL remained stable throughout the six weeks.
The researchers concluded that use of the smartphone app in this patient population was feasible and had a possible effect on QoL and therefore was ‘warranted for this population‘.
The app will be implemented across all five Danish regions in the new year, with the developers hoping that international expansion will be possible in future.
Andreas Dam, CEO of Daman – the digital health company behind the app‘s development – said: ‘Our work in co-creation with patients has been pivotal in creating a space that not only provides technological solutions but also emotional support for young cancer patients.‘
19th November 2023
The presence of haemoglobin in the skin’s epidermis has been discovered for the first time, offering insight into the skin’s defence mechanisms against ageing and cancer, researchers have announced.
Published in the Journal of Investigative Dermatology, a team from the Keio University School of Medicine in Tokyo sought to determine candidates for unidentified molecules involved in the barrier mechanism by analysing highly expressed genes in the upper epidermis of both humans and mice.
They conducted a comparative transcriptome analysis of the whole and upper epidermis, both of which were enzymatically separated as cell sheets from human and mouse skin.
They found that the messenger RNA level of haemoglobin α, an oxygen carrier in erythroid cells, was enriched in the upper epidermis compared with the levels in the whole epidermis.
To confirm their findings, they then used immunostaining to visualise the presence of haemoglobin α protein in keratinocytes of the upper epidermis.
The researchers also discovered that epidermal haemoglobin was upregulated by oxidative stress and inhibited the production of reactive oxygen species in human keratinocyte cell cultures.
As haemoglobin binds gases such as oxygen, carbon dioxide, and nitric oxide, and is also an iron carrier via the heme complex, it is therefore thought that epidermal haemoglobin is a prime candidate for antioxidant activity and potentially other roles in barrier function.
Professor Masayuki Amagai was lead investigator of the study and professor in the Department of Dermatology at Tokyo’s Keio University School of Medicine, and the Laboratory for Skin Homeostasis at the RIKEN Center for Integrative Medical Sciences, Yokohama.
He said: ’Our findings suggest that haemoglobin α protects keratinocytes from oxidative stress derived from external or internal sources such as UV irradiation and impaired mitochondrial function, respectively.
’Therefore, the expression of haemoglobin by keratinocytes represents an endogenous defence mechanism against skin aging and skin cancer.’
Professor Amagai added: ’Previous studies have identified the expression of various genes with protective functions in keratinocytes during their differentiation and formation of the outer skin barrier.
’However, other barrier-related genes escaped prior detection because of difficulties obtaining adequate amounts of isolated terminally differentiated keratinocytes for transcriptome analysis.’
11th October 2023
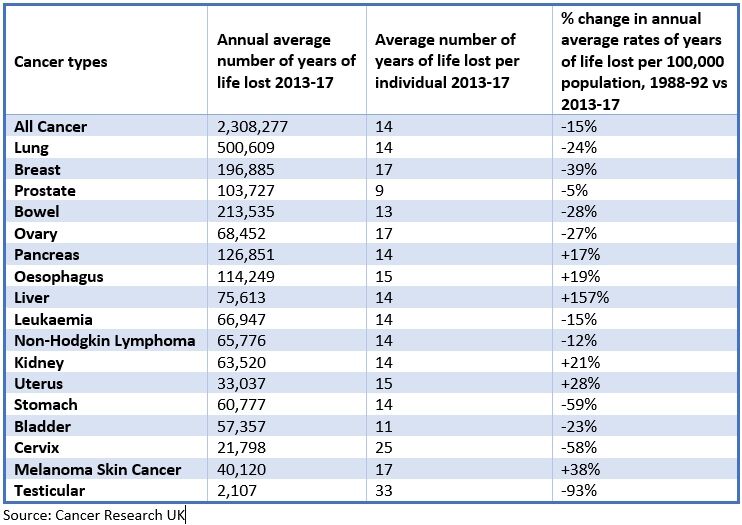
More than 2.3 million years of life are lost to cancer every year in the UK, but rates fell during the period of analysis due to advances in research and innovation, according to a new study from Cancer Research UK.
Researchers from King’s College London and Queen Mary University of London used the age at which cancer patients died from their disease and average life expectancy for the general population to estimate how many years were lost to cancer between 1988 and 2017.
The first statistical analysis of its kind and published in the British Journal of Cancer, the study found that overall years of life lost per year to all cancers combined has risen since the 1980s, largely due to the growing population.
Between 2013 and 2017, there annual average number of years of life lost was 2,308,277 for all cancers, up from 2,167,348 in 1988-92. However, when comparing the change in annual average rates of years of life lost per 100,000 population between 1988-92 and 2013-17, the average lost per individual had decreased for all cancers by 15%.
In 2013-17, more than 500,000 years of life – around a fifth of the total – were from lung cancer, which the researchers put down to its high diagnosis rates and poor survival. A total of 213,535 years of life were lost to bowel cancer each year and 196,885 to breast cancer.
When comparing the two time periods, lung, bowel and breast cancer rates were -24%, -28% and -39% respectively. The biggest decreases in these rates were seen in stomach (-59%) and cervical (-58%) cancers.
As testicular cancer is less common, a smaller number of lost years were seen overall, however its personal impact was substantial with an average of 33 lost years of life per individual. Though relatively few people die from the disease, survival is high and those who do die are usually younger.
In addition, while prostate cancer makes up 26% of cases amongst men, it only accounts for 9% of annual years of life lost due to the older age people are usually diagnosed and the relatively high survival rates.
Dr Judith Offman, who led the work at King’s College London and is now senior lecturer in cancer prevention and early detection at Queen Mary University of London, said: ‘This analysis allows us to see the impact cancer has on patients and their families, and the precious time that is lost as a result. Measuring years of life lost over a 30-year period provides a different lens to evaluate where health policies and advances in treatment have worked and highlight areas where more needs to be done.
‘Research like this is instrumental in helping leaders in health and politics make the best decisions for patients and their loved ones.’
This comes ahead of the publication of Cancer Research UK’s Manifesto for Cancer Research and Care, which is expected in the autumn and will outline exactly what it believes the UK Government can do to help beat cancer.
This will include driving progress in the prevention, diagnosis and treatment of cancer, investment in research and innovation, and addressing chronic staff and equipment shortages within the NHS.
30th August 2023
Young men with a higher level of cardiorespiratory fitness have a significantly lower risk of developing several cancers in later life, according to the findings of a new study published in the British Journal of Sports Medicine.
It is already known that aerobic exercise induces interleukin-6 and suppresses a marker of DNA damage, which may account for a protective role in colon cancer. But whether being fit could reduce the risk of developing cancer in later life is far less clear.
For the recent study, Swedish researchers set out to assess the associations between cardiorespiratory fitness in young men and the incidence of site-specific cancer.
They turned to data held on men who underwent military conscription between 1968 and 2005 and for whom cardiorespiratory function was assessed by maximal aerobic workload cycle test at conscription.
The men’s level of fitness was then categorised as low, moderate or high, and those who received a cancer diagnosis before or within five years after the military conscription were excluded from the analysis.
The team included 1,078,000 men, of whom 6.9% subsequently developed cancer in at least one site during a mean follow-up of 33 years.
A higher cardiorespiratory fitness was linearly associated with a significantly lower risk of developing nine different cancers. This included cancer in the head and neck (Hazard ratio, HR = 0.81), oesophagus (HR = 0.61), stomach (HR = 0.79), pancreas (HR = 0.88) and liver (HR = 0.60).
In contrast, a higher cardiorespiratory fitness significantly increased the risk of being diagnosed with prostate cancer (HR = 1.07) and malignant skin cancer (HR = 1.31).
While it is an observational study and no firm conclusions can be drawn about cause and effect, the researchers suggested that the findings strengthened the incentive for promoting interventions aimed at increasing cardiorespiratory fitness in younger people.
3rd May 2023
NHS England has tasked hospitals with turning around diagnostic test results for suspected cancer within 10 days.
Hundreds of patients who have been referred under the urgent pathway will receive faster news about whether they have cancer or not helping to reduce anxiety and start treatment more quickly, NHS England said.
A letter sent to local health leaders has also asked teams to prioritise diagnostic tests like MRI scans for cancer in community diagnostic centres (CDCs) or to free up capacity within hospitals by moving elective activity into the centres.
Earlier this month, figures showed more than 42% of patients are waiting more than 62 days for their first cancer treatment from urgent GP referral.
It follows a report from the Public Accounts Committee in March which warned that cancer waiting times are at their worst ever level and NHS England was unlikely to meet its recovery target of moving back to 85% treated within 62 days of referral.
But the latest figures did show some improvement in two week wait times from the previous month with 86% of people seen by a specialist within a fortnight of urgent referral up from 81%.
In February, NHS England said it achieved the faster diagnosis standard for suspected cancer for the first time, with three quarters of those referred receiving a definitive diagnosis or all clear within 28 days – 171,453 people.
There has been high demand for services with up to one in four GP referrals a month for cancer.
In March 2022 to Feb 2023, 470,000 more people were checked for cancer compared with the same period before the pandemic, the figures show.
There are now 105 CDCs in place and offering a ‘one stop shop’ for tests, NHS England confirmed.
Dame Cally Palmer, NHS national director for cancer, said: ‘It is a testament to the hard work of NHS staff that we are seeing and treating record numbers of patients for cancer, and have made significant progress bringing down the backlog and achieving the target for diagnosing three quarters of people within 28 days – all despite huge demand and pressures on the system.
‘Fortunately, the vast majority of suspected cancer patients waiting for a diagnostic test will not have cancer, but for those waiting it can be a very anxious time, so we are asking trusts to aim for a 10-day turnaround time between GP referral and tests results for patients – so we can get people the all-clear faster, or in some cases ensure patients diagnosed with cancer are able to start treatment sooner.’
Professor Mike Osborn, president of the Royal College of Pathologists, said: ‘We welcome the announcement of support for pathology services which will help our members provide the quicker diagnoses that patients need.
‘Pathologists have long asked for improvements in digital pathology and infrastructure to help them provide better patient care. We fully support this initiative and the fresh focus on pathology which it should provide will, we hope, make a real difference to patients.’
This news story was originally published by our sister publication Pulse.
19th April 2023
Major depressive disorder affects around 14.3% of patients with cancer. Advances in psycho-oncology show that psychotropic drugs are effective for cancer patients. There is some data suggesting that psilocybin therapy for cancer patients improves depressed mood and anxiety. Whether this effect occurs in cancer patients with a diagnosis of major depressive disorder (MDD) is not clear.
In the current study, researchers gave adult cancer patients and a diagnosis of MDD, a single 25 mg dose of psilocybin. Individuals were divided into cohorts and had a single group preparation session and two group integration sessions. Therapeutic care was provided throughout the study using the 1:1 model of psychological support. Researchers assessed the safety of psilocybin therapy and the effect on depression with the Montgomery Asberg Depression Rating Scale (MADRS).
Psilocybin therapy outcomes
The study had 30 patients, all with MDD and assessments carried out after 8 weeks. The study observed a robust and significant decrease in MADRS score (p < 0.0001) at week 8 with a ≥ 50% decrease in the MADRS score in 24 patients at week 8. Half of the group had complete remission of depression symptoms (a MADRS score < 10) after 7 days which was still present at week 8.
Self-reported depressive symptom scores were 48% lower at week 8. The Maudsley visual analogue scale was 53% lower at week 8. There were no treated-related serious adverse effects reported.
Citation
Agrawal M et al. Assessment of Psilocybin Therapy for Patients With Cancer and Major Depression Disorder. JAMA Oncol 2023
21st December 2022
Cannabidiol (CBD) oil given to patients with advanced cancer receiving palliative care provided no additional benefit to that care according to the findings of a randomised trial by Australian researchers.
Although there have been several advances in medical care, a proportion of patients with advanced cancer still experience substantial symptom distress. The use of palliative care seeks to improve both symptom control and quality of life but despite this, some symptoms can be difficult to control, necessitating more effective medications. Both cannabis and cannabinoid drugs containing cannabidiol, are widely used to treat disease or alleviate symptoms. However, a 2015 meta-analysis concluded that whilst there was moderate-quality evidence to support the use of cannabinoids for the treatment of chronic pain and spasticity, there was low-quality evidence suggesting that cannabinoids were associated with improvements in nausea and vomiting due to chemotherapy. In a feasibility study, Australian researchers examined the use of global symptom burden measures to assess the response to medicinal cannabis with both cannabidiol and tetrahydrocannabinol. They concluded that doses of both cannabidiol and tetrahydrocannabinol were generally well tolerated and that the outcome measure of total symptom distress was promising as a measure of overall symptom benefit.
Based on the these early and promising findings, the same group undertook a randomised trial to determine whether cannabidiol oil could improve symptom distress in patients with advanced cancer receiving palliative care. They included adult participants with advanced cancer and symptom distress which was measured using the Edmonton Symptom Assessment Scale [ESAS]. Participants received titrated CBD oil 100 mg/mL, 0.5 mL once daily to 2 mL three times a day, or matched placebo for 28 days. The ESAS scale is designed to rate the intensity of nine common symptoms experienced by cancer patients, including pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being and shortness of breath. For the trial participants, the inclusion criterion was an ESAS score greater than or equal to 10/90. The primary outcome was set as the total ESAS symptom distress score (TSDS) at day 14, with a response defined as a decrease greater than or equal to, 6 at day 14.
Cannabidiol oil and symptom distress
A total of 58 patients receiving CBD and 63 placebo, reached the primary analysis point (i.e., day 14) and the median dose of participant-selected CBD was 400 mg per day.
The unadjusted change in TSDS from baseline -6.2 for the placebo group and -3.0 for those receiving CBD and this difference was non-significant (p = 0.24). Equally, there was no significant difference in proportion of responders (placebo = 58.7% and CBD = 44.8% p = 0.13).
In fact, during the study, all components of the ESAS improved (that is, reduced) over time with no difference between the placebo and CBD arms. In addition, there was no detectable effect of CBD on quality of life, depression, or anxiety. Overall, most participants reported feeling better (53% CBD vs 65% placebo) or much better (70% CBD and 64% placebo) by day 14.
The authors concluded that CBD oil did not add value to the reduction in symptom distress provided by specialist palliative care alone.
Citation
Hardy J et al. Phase IIb Randomized, Placebo-Controlled, Dose-Escalating, Double-Blind Study of Cannabidiol Oil for the Relief of Symptoms in Advanced Cancer (MedCan1-CBD). J Clin Oncol 2022
12th May 2022
Cancer patients with COVID-19 have been found to be at a greater risk of hospitalisation and 30-day all-cause mortality compared to those without the disease according to the results of a study by a US team from Texas.
The presence of cancer has become a recognised factor that is associated with a higher risk for severe outcomes in those infected with COVID-19 and which is largely due to the presence of a compromised immune system.
During the early course of the pandemic, studies observed that a higher proportion of cancer patients infected with COVID-19 were both hospitalised and subsequently died, compared to those without the disease. In contrast, however, other studies have suggested that cancer and non-cancer patients have comparable COVID-19 outcomes after adjusting for age, sex, and comorbidity.
Furthermore, the impact of factors such as cancer treatments, different cancer types on COVID-19 related outcomes has been less well studied.
For the present study, the US researchers examined the association between cancer-specific characteristics and COVID-19 outcomes. They turned to the Optum de-identified COVID-19 electronic health record, which is derived from over 700 hospitals and 7000 clinics across the USA. Using these data, the researchers examined the outcome of those with a laboratory confirmed COVID-19 and a recorded cancer diagnosis.
The primary objective was to determine the effect of cancer on COVID-19 outcomes including 30-day all-cause mortality, hospitalisation, intensive care unit (ICU) admission and ventilator use. These outcomes were also analysed by the nature and type of cancer in comparison to patients without cancer.
The authors the explored if there were any other specific factors in those with cancer which impacted on COVID-19 outcomes.
Cancer patient with COVID-19 and related outcomes
A total of 271,639 patients with confirmed COVID-19 of whom 18,460, with a mean age of 66 years (45.3% male) had a cancer diagnosis were analysed. Among those with cancer, 8034 patients had a history of cancer for longer than 12 months and 10,426 had a more recent diagnosis, i.e., within 1 year before COVID-19.
30-day all-cause mortality was more than three times higher among those with cancer (6.8% vs 1.9%) compared to non-cancer patients. After adjustment for age, sex, ethnicity and risk factors, the presence of cancer was associated with a 7% higher risk of death (relative risk, RR = 1.07, 95% CI 1.01 – 1.14, p = 0.028) compared to those without the disease.
Similarly, there was a 4% higher risk of hospitalisation (RR = 1.04, 95% CI 1.01 – 1.07, p = 0.006). When comparing the duration of cancer, those with a recent diagnosis had both a significant (p < 0.001) increased risk of mortality (RR = 1.17) and hospitalisation (RR = 1.10) although this risk was non-significant for those who had cancer for much longer.
There was also an increased mortality risk for those with recent metastatic (RR = 2.09), solid tumour (RR = 1.12) and haematological (RR = 1.48) cancers compared with those without the disease. Individual cancers with a significantly elevated risk were leukaemia (RR = 1.58), liver (RR = 2.46), lung (RR = 1.85) and pancreatic (RR = 1.94).
When exploring the factors related to COVID-19 mortality in those with recent cancer, both chemotherapy (RR = 1.37) and radiotherapy (RR = 1.83) within 3-months before COVID-19, were significantly associated with a higher risk of death as was increasing age (i.e., > 75 years) (RR = 6.69).
In addition, the only significant co-morbidities were cardiovascular disease (RR = 1.72), diabetes (RR = 1.39) and renal disease (RR = 1.51).
Citation
Kim Y et al. Characterizing cancer and COVID-19 outcomes using electronic health records PLoS One 2022