This website is intended for healthcare professionals only.
Take a look at a selection of our recent media coverage:
8th December 2022
16th September 2022
For many, EuroPCR 2022 marked the return of face-to-face learning and discussions with peers from across the globe. Many interactive sessions were held across the week in May, with excellent presentations from top-class clinicians. This report summarises some key sessions on transcatheter aortic valve implantation (TAVI), valve-in-valve (ViV) interventions and optimisation of such procedures.
Valve-in-valve: the journey starts with the first prosthesis
To understand the impact of valve-in-valve interventions on lifetime management
To learn about clinical considerations and the procedural technique of TAVI-in-TAVI procedures
To appreciate the potential pitfalls of such procedures and how to mitigate them
To discuss remaining uncertainties in the diagnosis and management of aortic valve disease
To appreciate available and building evidence for patients with asymptomatic severe aortic stenosis (AS) and symptomatic moderate AS
To understand challenges and options for interventional treatment of pure native aortic regurgitation
Learnings from an optimised TAVI patient’s journey
To understand best practices related to referral and in-hospital patient pathways
To discover how an optimised TAVI care pathway offers excellent clinical safety and quality of life and leads to healthcare economics benefits
To learn how and which TAVI procedural aspects can be optimised for the greater good of the patient and the healthcare system
Key takeaways
Occlusion of the coronary arteries is a risk of valve-in-valve (ViV) that must be mitigated by thorough procedural planning, with the first transcatheter heart valve (THV) having a large influence
For patients with longer life expectancy, the implantation of a second TAVI should be included in the planning of the first TAVI implementation
ViV procedure volumes are expected to grow in the future as we will see patients implanted with first generation bioprosthesis returning
Valve-in-valve: what does it mean for lifetime management?
The first talk of the session was from Dr. Radoslaw Parma (Interventional Cardiologist, Medical University of Silesia).
A recent report from the German Heart Surgery Registry showed that the number of mechanical prostheses has reduced considerably over the last decade, and a shift towards biological implantation has been seen.1 Dr. Parma explained this is likely due to increasing comorbidities in patients, resulting in contraindications to anticoagulants, and further predicts that, in the majority of countries, the number of TAVI procedures will soon exceed those of surgical aortic valve replacement (SAVR).
From 2011 to 2019 there was a large change in the surgical risk scores for patients receiving TAVI, moving from higher/intermediate risk to low-risk patients.2 In low-risk patients receiving TAVI, there is a high chance of needing ViV TAVI in the future. ViV TAVI demonstrated better early outcomes, including reduced 30-day mortality, risk of bleeding, and hospital length of stay.3 On this basis, Dr. Parma suggested that the future for primary SAVR patients may be TAVI-in-SAVR instead of reoperation, meaning consideration of the initial valve type and its failure, in regard to the patient’s anatomy, is key.
Finally, Dr. Parma discussed the difficulties of future coronary artery disease (CAD) in younger, low-risk patients. The SOURCE 3 registry showed that >3% of patients required intervention due to CAD following TAVI; for most, successful coronary angiography was achieved, however successful percutaneous coronary intervention (PCI) was sometimes inhibited.4 5
TAVI-in-TAVI: What is important?
Prof. Giuseppe Tarantini (Interventional Cardiologist, University of Padua) was the next to speak in this session on reintervention for high-, intermediate-, and low-risk patients.
| High/intermediate/low risk (>75 years old) | High/intermediate risk (<75 years old) | Low risk (<75 years old) | |
|---|---|---|---|
| Life expectancy following SAVR | 5.7-8.2 years 6 | 5.6-8.4 years 6 | 12.5-16.2 years 6,7 |
In 80-year-old patients, TAVI explantation has 30-day and 1-year mortality risk of 12.3% and 20.8%, respectively.9 While in 72-year-old patients, TAVI explantation has 30-day and 1-day mortality risk of 13.1% and 28.5%, respectively.10
Prof. Tarantini went on to explain the importance of THV orientation and commissural alignment in regard to patient’s anatomy. Without needing any commissural alignment, the SAPIEN 3 valve achieved 95% of selective coronary access, whereas it was 71% for aligned supra-annular THVs and 46% for misaligned supra-annular THVs (P<0.001).11
Prof. Tarantini identified four factors to consider for coronary obstruction in redo-TAVI:
Risk plane
Valve to aorta (VTA) distance
Sinus sequestration
To finish the session, Prof. Christian Hengstenberg (Interventional Cardiologist, Medical University of Vienna) showed a case presentation of a TAVI-in-TAVI in his hospital. This Live-in-a-box case illustrated the concepts described by the Faculty (thorough planning including maintaining access to coronaries): a SAPIEN 3 valve was successfully implanted into another SAPIEN 3 valve, with frame alignment, decreasing the mean gradient from 44 mmHg to 8 mmHg without any complications.
Key takeaways
Aortic stenosis (AS) is a disease continuum and can be classified by many forms and degrees
Grading AS patients by extent of cardiac damage (e.g., left ventricular damage), instead of mild, moderate and severe may be a more objective way to classify patients
There are also intervention options for pure AR, despite being a challenging patient population
Aortic valve stenosis of uncertain severity
Prof. Lars Sondergaard (Consultant Cardiologist, Rigshospitalet) began the first session, presenting a symptomatic case of a 79-year-old female showing very borderline severe AS on several diagnostic tests, including echocardiography, invasive coronary angiography, and computed tomography (CT).
The key takeaway from this case was the importance of diligently assessing the patient baseline characteristics and perform a fully comprehensive assessment. Even with borderline grade (moderate to severe AS) anatomy, it is important to consider that the patient is highly symptomatic so relieving this should be a priority when deciding to intervene.
Truly asymptomatic severe AS
Next, Dr. Philippe Généreux (Interventional Cardiologist, Morristown Medical Centre) presented a case of a 78-year-old male with clear severe AS and suitable anatomy for both TAVI and SAVR, including a highly calcified tricuspid valve; yet he was asymptomatic.
Dr. Généreux estimated that there was a 20 – 30% chance of this patient needing intervention within a year and a low risk of sudden death yet suggested that early intervention before onset of symptoms can prevent mortality and left ventricle (LV) dysfunction. The panel consensus was that patient preference is key here; CT scan should be performed early to ascertain the complexity of the intervention to ensure both efficacy and safety.
Symptomatic moderate AS
Dr. Nicole Karam (Interventional Cardiologist, Hôpital Européen Georges-Pompidou) presented a case of an 85-year-old male who has moderate AS, but is highly symptomatic.
Dr. Généreux explained a new method to classify patients with AS. In addition to grading by mild, moderate, and severe, this can be done by extent of cardiac damage.12 These included Stage 1 with LV damage, up to Stage 4 with right ventricular (RV) damage; these stages were seen to correlate with risk of mortality.12
When comparing this stage system with the traditional grades, Dr Généreux emphasised that there are two interesting categories of patients:
Severe AS who classed as stage 0 (no cardiac damage)
Moderate AS who classed as stage 0 up to stage 4
Dr. Généreux suggested this may be a more objective way to classify patients and to alleviate the need to specify symptoms. So, in the future it may be stage of cardiac damage that could be incorporated into future recommendations for risk stratification.
There are number of ongoing randomised control trials assessing the efficacy of TAVI with the SAPIEN 3 valve for the aforementioned controversial patient cohorts. These include the Early TAVR trial focusing on asymptomatic patients with severe calcified AS, as well as the TAVR Unload trial and PROGRESS trial focusing on moderate AS patients.13–15
Anatomical and procedural challenges of patients with pure native aortic regurgitation
Dr. Nicolo Piazza (Interventional Cardiologist, McGill University Health Centre) presented the next session on aortic regurgitation (AR). AR is morphologically classified by 2 mechanisms (1) abnormal motion of the leaflets that are prolapsing or retracted, and (2) enlarged aortic root or ascending aorta.16
Around 1 in 10 patients with any valvular heart disease has some severity of AR.17 For TAVI, the most common aetiologies are degenerative (50%), rheumatic (15%), and congenital (15%), whereas the most common indications were degenerative (63%), post-endocarditis (8%), and aortic aneurysm (11%).17,18
Dr. Piazza went on to explain that the challenges in performing TAVI for pure AR can lie with the patient, the device or the operator.
| Patient | Device | Operator |
|---|---|---|
|
|
|
The little experience from the operator comes from a very low number of TAVIs being performed for AR. An average of only ~8 cases per centre per year were performed; even in centres performing >1000 TAVIs each year, a maximum of 17 TAVIs for pure AR was reported.18
Key takeaways
Severe aortic stenosis (AS) prevalence is increasing, with many patients remaining undiagnosed or untreated
Delays in the patient pathway have a strong impact on morbidity and mortality
It is key that the Heart Team recommendation is discussed with the patient who can then make an informed decision
A streamlined evidence-based TAVI pathway, such as the Edwards Benchmark program, can provide excellent safety outcomes and conserved hospital resources, enabling more patients to access high-quality care
Today’s challenges
Dr. Mick Ozkor (Interventional Cardiologist, Barts Heart Centre) began the session by presenting data showing that, of 10,795 patients, 57% had an indication or potential indication for AVR, of whom only 48% received an AVR.19 The consequence of this delayed referral is increased mortality rates, particularly in those patients with impaired ejection fraction.19 Even if patients are referred, prolonged waiting time is associated with increased mortality.20
Refer better, treat faster
The UK performs significantly lower numbers of TAVI procedures compared with other European countries, likely due to commissioning constraints and significantly fewer implanting centres.21 This under-provision has resulted in geographical inequity, which, in turn, causes waiting times as long as 20 weeks from time of referral.21
In this section, Dr. Joanne Shannon (General Cardiologist, Frimley Park Hospital) discussed how she has optimised the TAVI pathway in her hospital.

Compared to the national median time from referral to TAVI of 20 weeks, Frimley Park referral time was 12 weeks as a result of this optimised pathway. Dr. Shannon suggested further improvements of:
Better access to CT imaging
Better access to catheter lab slots for angiography/PCI
Daily triaging of referrals
Weekly telephone reviews of all patients on the waiting list to pre-empt and initiate change in priority status
Regular meetings between local TAVI nurse and surgical centre TAVI coordinator
Better access to surgeons
Attendance of implanting cardiologists at all Heart Team meetings, where possible
Increasing catheter lab capacity or expand to non-surgical centres
Patient’s informed decision and expected outcomes
Prof. Eric Durand (Interventional Cardiologist, University Hospital of Rouen) explained that making an informed decision with a patient must include the clinical, anatomical, and procedural information as well as the life expectancy and relative risk of the procedure. The panel concluded that, for borderline patients, discussions should be with both an interventional cardiologist and a surgeon.
Key practices for optimisation – Kiel Centre’s experience
This session went through the best practices associated with the Edwards Benchmark program. This includes some very important steps:
Structured screening programme
TAVI coordinator
Minimalist TAVI practices, local anaesthesia
Early mobilisation
The ambitious safety goals include 30-day rates of 1% mortality, 1% stroke, 6% permanent pacemaker, 4% cardiac readmissions and 1% major vascular complications, as well as 80% next day discharge.22–24
Optimised peri- and post-TAVI procedural steps – Charité Centre’s experience
Prof. Henryk Dreger (Interventional Cardiologist, Charité Universitätsmedizin Berlin) went on to explain key pre-, peri-, and post-procedural steps which optimise TAVI in 2022.
| Pre-procedure | Peri-procedure | Post-procedure |
|---|---|---|
|
|
|
Prof. Dreger finished by presenting research supporting the safety of early discharge in France, Italy, the Netherlands, UK, and North America.22,25,26 In France, multicentre studies demonstrated lower or similar all-cause mortality post-TAVI up to 3 years following early discharge.25,26 In the FAST-TAVI trial performed in Italy, the Netherlands and the UK, patients appropriately discharged early had significantly lower risk of the primary endpoint (7% vs 26.4%, p<0.001), which was reflected in some of its components: stroke, permanent pacemaker implantation, major vascular complications and major/life-threatening bleeding.22 In North America, the 3M TAVI clinical pathway ensured early discharge with excellent outcomes.24
Closing statement
The sessions summarised in this report walk through some important considerations for TAVI for clinicians and hospital decision makers alike. With the indication for TAVI expanding beyond patients at high surgical risk to those as intermediate and low risk, valve considerations based on future ViV TAVI, patient preference, and increasing capacity for TAVI are becoming increasingly important. The ambitious goals of the Edwards Benchmark program can optimise the TAVI pathway and should be considered by hospitals across Europe.
Session references
1. Valve-in-valve: the journey starts with the first prosthesis. Presented at EuroPCR 2022 on 17th May.
2. Emerging indications for TAVI. Presented at EuroPCR 2022 on 17th May.
3. Learnings from an optimised TAVI patient’s journey. Presented at EuroPCR 2022 on 18th May.
Speaker references from sessions
References from speakers in ‘Valve-in-valve: the journey starts with the first prosthesis’
1. Beckmann A, Meyer R, Lewandowski J, Markewitz A, Gummert J. German Heart Surgery Report 2020: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg. 2021;69(4):294-307. doi:10.1055/s-0041-1730374
2. Carroll JD, Mack MJ, Vemulapalli S, et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2020;76(21):2492-2516. doi:10.1016/j.jacc.2020.09.595
3. Sá MPBO, van den Eynde J, Simonato M, et al. Valve-in-Valve Transcatheter Aortic Valve Replacement Versus Redo Surgical Aortic Valve Replacement. JACC Cardiovasc Interv. 2021;14(2):211-220. doi:10.1016/j.jcin.2020.10.020
4. Tarantini G, Nai Fovino L, le Prince P, et al. Coronary Access and Percutaneous Coronary Intervention Up to 3 Years After Transcatheter Aortic Valve Implantation With a Balloon-Expandable Valve. Circ Cardiovasc Interv. 2020;13(7):e008972. doi:10.1161/CIRCINTERVENTIONS.120.008972
5. Tarantini G, Nai Fovino L. Lifetime Strategy of Patients With Aortic Stenosis: The First Cut Is the Deepest. JACC Cardiovasc Interv. 2021;14(15):1727-1730. doi:10.1016/j.jcin.2021.06.029
6. Martinsson A, Nielsen SJ, Milojevic M, et al. Life Expectancy After Surgical Aortic Valve Replacement. J Am Coll Cardiol. 2021;78(22):2147-2157. doi:10.1016/j.jacc.2021.09.861
7. Glaser N, Persson M, Jackson V, Holzmann MJ, Franco-Cereceda A, Sartipy U. Loss in Life Expectancy After Surgical Aortic Valve Replacement: SWEDEHEART Study. J Am Coll Cardiol. 2019;74(1):26-33. doi:10.1016/j.jacc.2019.04.053
8. Tarantini G, Nai Fovino L, le Prince P, et al. Coronary Access and Percutaneous Coronary Intervention Up to 3 Years After Transcatheter Aortic Valve Implantation With a Balloon-Expandable Valve. Circ Cardiovasc Interv. 2020;13(7):e008972. doi:10.1161/CIRCINTERVENTIONS.120.008972
9. Percy ED, Harloff MT, Hirji S, et al. Nationally Representative Repeat Transcatheter Aortic Valve Replacement Outcomes: Report From the Centers for Medicare and Medicaid Services. JACC Cardiovasc Interv. 2021;14(15):1717-1726. doi:10.1016/j.jcin.2021.06.011
10. Bapat VN, Zaid S, Fukuhara S, et al. Surgical Explantation After TAVR Failure: Mid-Term Outcomes From the EXPLANT-TAVR International Registry. JACC Cardiovasc Interv. 2021;14(18):1978-1991. doi:10.1016/j.jcin.2021.07.015
11. Tarantini G, Nai Fovino L, Scotti A, et al. Coronary Access After Transcatheter Aortic Valve Replacement With Commissural Alignment: The ALIGN-ACCESS Study. Circ Cardiovasc Interv. 2022;15(2). doi:10.1161/CIRCINTERVENTIONS.121.011045
References from speakers in ‘Emerging indications for TAVI’
12. Généreux P, Pibarot P, Redfors B, et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur Heart J. 2017;38(45):3351-3358. doi:10.1093/eurheartj/ehx381
13. ClinicalTrials.gov. EARLY TAVR: Evaluation of TAVR Compared to Surveillance for Patients With Asymptomatic Severe Aortic Stenosis (EARLY TAVR). Published 2022. Accessed July 26, 2022. https://clinicaltrials.gov/ct2/show/NCT03042104
14. ClinicalTrials.gov. Transcatheter Aortic Valve Replacement to UNload the Left Ventricle in Patients With ADvanced Heart Failure (TAVR UNLOAD). Published November 1, 2021. Accessed July 26, 2022. https://clinicaltrials.gov/ct2/show/NCT02661451
15. ClinicalTrials.gov. PROGRESS: Management of Moderate Aortic Stenosis by Clinical Surveillance or TAVR (PROGRESS). Published July 8, 2022. Accessed July 26, 2022. https://clinicaltrials.gov/ct2/show/NCT04889872
16. Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303-371. doi:10.1016/j.echo.2017.01.007
17. Iung B. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24(13):1231-1243. doi:10.1016/S0195-668X(03)00201-X
18. Sawaya FJ, Deutsch MA, Seiffert M, et al. Safety and Efficacy of Transcatheter Aortic Valve Replacement in the Treatment of Pure Aortic Regurgitation in Native Valves and Failing Surgical Bioprostheses: Results From an International Registry Study. JACC Cardiovasc Interv. 2017;10(10):1048-1056. doi:10.1016/j.jcin.2017.03.004
References from speakers in ‘Learnings from an optimised TAVI patient’s journey’
19. Li SX, Patel NK, Flannery LD, et al. Trends in Utilization of Aortic Valve Replacement for Severe Aortic Stenosis. J Am Coll Cardiol. 2022;79(9):864-877. doi:10.1016/j.jacc.2021.11.060
20. Malaisrie SC, McDonald E, Kruse J, et al. Mortality while waiting for aortic valve replacement. Ann Thorac Surg. 2014;98(5):1564-1570; discussion 1570-1. doi:10.1016/j.athoracsur.2014.06.040
21. Ali N, Faour A, Rawlins J, et al. “Valve for Life”: tackling the deficit in transcatheter treatment of heart valve disease in the UK. Open Heart. 2021;8(1). doi:10.1136/openhrt-2020-001547
22. Barbanti M, van Mourik MS, Spence MS, et al. Optimising patient discharge management after transfemoral transcatheter aortic valve implantation: the multicentre European FAST-TAVI trial. EuroIntervention. 2019;15(2):147-154. doi:10.4244/EIJ-D-18-01197
23. Lauck SB, Wood DA, Baumbusch J, et al. Vancouver Transcatheter Aortic Valve Replacement Clinical Pathway: Minimalist Approach, Standardized Care, and Discharge Criteria to Reduce Length of Stay. Circ Cardiovasc Qual Outcomes. 2016;9(3):312-321. doi:10.1161/CIRCOUTCOMES.115.002541
24. Wood DA, Lauck SB, Cairns JA, et al. The Vancouver 3M (Multidisciplinary, Multimodality, But Minimalist) Clinical Pathway Facilitates Safe Next-Day Discharge Home at Low-, Medium-, and High-Volume Transfemoral Transcatheter Aortic Valve Replacement Centers: The 3M TAVR Study. JACC Cardiovasc Interv. 2019;12(5):459-469. doi:10.1016/j.jcin.2018.12.020
25. Durand E, le Breton H, Lefevre T, et al. Evaluation of length of stay after transfemoral transcatheter aortic valve implantation with SAPIEN 3 prosthesis: A French multicentre prospective observational trial. Arch Cardiovasc Dis. 2020;113(6-7):391-400. doi:10.1016/j.acvd.2019.11.010
26. Durand E, Avinée G, Tron C, et al. Analysis of Length of Hospital Stay after Transfemoral Transcatheter Aortic Valve Implantation: Results from the FRANCE TAVI (FRench Transcatheter Aortic Valve Implantation) Registry. Structural Heart. 2019;3:204. doi:10.1080/24748706.2019.1588542
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, SAPIEN, SAPIEN 3, and Edwards Benchmark are trademarks or service marks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
© 2022 Edwards Lifesciences Corporation. All rights reserved. PP–EU-4713 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
19th August 2022
2nd August 2022
The European Society of Cardiology (ESC) Atlas undertakes an annual survey to obtain data on cardiovascular procedure rates across ESC member countries, and the variation in human and capital resources that underpin this variation.1 As contemporary treatment for cardiovascular disease (CVD) becomes increasingly technological, it is important to consider the accompanying financial burden and its impact on low- and middle-income countries. The ESC Cardiovascular Realities 2020 clearly depicts the three key elements of the survey: human resources, capital resources, and service delivery; all of which are strongly associated with national economic resource, and often irrespective of healthcare need.1
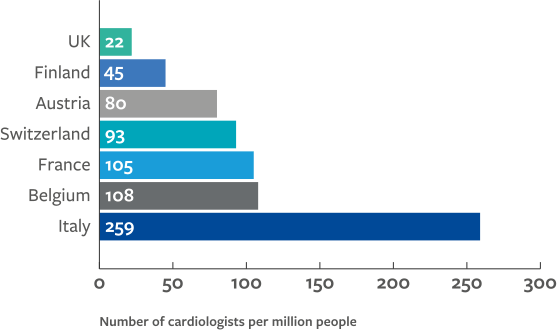
The disparities in human and capital resources between low-, middle-, and high-income countries underpins the same inequalities in delivery of cardiovascular treatment. The ESC Atlas survey highlights considerably less availability of services in middle- compared to high-income countries, including for invasive coronary angiography (ICA), PCI, structural heart intervention, ablation and rhythm management devices, cardiac surgery, and heart transplantation and left ventricular access devices (LVAD).
Key findings:
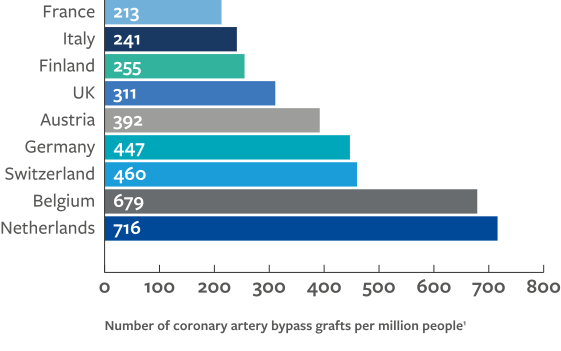
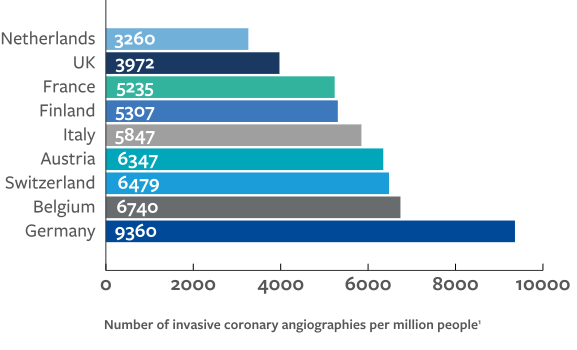
These data indicate a strong association between national economic resource and availability of provisions and treatment since high-income countries generally perform the greatest number of procedures. However, outliers from this trend may highlight other factors that are at play such as:
The association between gross national income (GNI) per capita and rates of diagnostic cardiac catheterisations makes apparent the constraints that expensive novel technologies are putting on healthcare systems, particularly for low- and middle-income countries.2 The prevalence of ischaemic heart disease is seen to be inversely associated with GNI per capita, suggesting procedural rates in high-income countries are paradoxically high and are more likely to be associated with national prosperity than healthcare demand.2
While middle-income countries are generally under resourced, other factors must be responsible for the observed outliers. Inter-country variation in medical device costs may also influence availability of care, for instance coronary stents and pacemakers are more expensive in Italy and France than in Germany; and healthcare expenditure is higher in the US than the UK, yet differences in allocation of these costs may result in different, if not better, outcomes in the UK.3,4
Aside from economic concerns, there is also a considerable variation in patient populations across Europe. In patients with heart failure with preserved ejection fraction (HFpEF), those from Western Europe (e.g., Austria, Belgium, Finland, France, UK, Spain, Switzerland) were oldest and had highest prevalence of atrial fibrillation, while those in Central and Eastern Europe (e.g., Bulgaria, Croatia, Greece, Hungary, Poland, Russia, Turkey) were youngest and had the highest prevalence of coronary artery disease.5 As a result, Western Europe reported higher incidences of heart failure hospitalisation and CV-related death, which may explain the greater service delivery in these countries.1,5
For middle-income countries, unnecessary provision not only has negative outcomes where the patient selection is inappropriate but is also likely to deplete available resources.1 In the same way, risk factor modification may be a cost-effective strategy to reduce the cardiovascular burden.1 However, given the cardiovascular risk factors that are associated with socioeconomic gradients both within and between countries, such as smoking, it is unlikely that risk factor modification alone is sufficient to increase availability of cardiovascular care in middle-income countries.1
This content is sponsored by Edwards Lifesciences.
20th July 2022
Cost savings in transcatheter aortic valve implantation (TAVI) cohorts, compared to surgical aortic valve replacement (SAVR), are driven mainly by shorter length of stay, as well as fewer complications and required resources.1–3 Such findings support prioritisation of TAVI over SAVR in patients with severe AS for whom TAVI is suitable across low-, intermediate- and high- surgical risk.2,4 Minimalist TAVI (M-TAVI) should also be considered to take the cost saving further, without compromising efficacy or safety.5
Policy makers may consider such benefits to inform a holistic consideration including both medical and societal impact, instead of considering procedural, rehabilitation, and budget separately.1
TAVI was introduced as an alternative to SAVR for the treatment of severe symptomatic aortic stenosis (AS). Initially only considered for patients with inoperable disease or those at high risk of surgical mortality, TAVI is now an effective and safe option for patients at intermediate or low risk of surgical death.1–3
The benefits of TAVI stem further than solely clinical efficacy, with research in recent years showing the proven cost effectiveness, compared with SAVR. Initially, concerns arose of the higher procedural costs of TAVI, yet this is not consistently reported: while procedural costs of TAVI were reported at €3,317 higher per patient over a lifetime horizon than for SAVR in Italy, a recent study based in France confirmed lifetime intervention costs per person (without pacemaker) to be €8,139 compared to a higher €22,603 for SAVR.1–3
Upon closer inspection, the reported higher valve costs of TAVI were offset by the lower short-term non-procedural costs, such as rate of complications, time spent in intensive care unit (ICU) and overall length of hospitalisation.1,3 A US cost-effectiveness study based on data from the PARTNER 3 trial showed that despite the overall hospital index costs for TAVI being marginally higher, the non-procedural costs were $7,174 compared to $23,578 for SAVR; non-procedural costs included procedure duration, length of stay and discharge disposition.6 Consequently total 2-year costs for TAVI were $2,030 less than for SAVR.6
TAVI with the SAPIEN 3 valve shows a compelling value-based case for patients in France driven by lower long-term management costs of disabling stroke and treated atrial fibrillation (AF).2 Lifetime costs relating to disabling stroke and treated AF were €5,744 and €5,219 less than for SAVR, respectively.2
In Norway, TAVI with the SAPIEN 3 valve is seen to be dominant over surgery in the low-risk population, as it is more effective (gain of 0.05 quality adjusted life years (QALY)) and less costly (saving of NOK 35,000) over a 15-year horizon.7 This is consistent with findings from Italy, where lower complication rates drive cost-effectiveness of TAVI with the SAPIEN 3 valve. This lower rate of complications means that TAVI is cost-effective at a willingness-to-pay threshold of €30,000/QALY.1
In a US-based study, significantly lower rates of major bleeding contribute to the cost effectiveness of TAVI using SAPIEN 3 valve over SAVR in intermediate-risk patients.3 In this study, however, the lower overall non-procedural costs of TAVI are primarily driven by the total length of hospital stay, which was on average 6.3 days shorter.3 This was comprised of a 2.8-day shorter ICU stay, 3.5-day shorter non-ICU stay and 5.6 days shorter post-procedure stay, and thus a €23,035 cost saving.3
Findings from a Spanish public hospital also confirmed the shorter hospital stay following TAVI. This clinical benefit reflected a considerable reduction in cost from €8,263.10 per patient for SAVR to €3,709.90 per patient for TAVI.8
Staff costs are considerably lower following percutaneous TAVI compared to SAVR.8 In Spain, the staff allocated to TAVI were required for only 158 minutes per procedure, compared to 261 minutes for SAVR which incurred a cost saving of €537.50 per patient (€308.10 TAVI vs. €845.60 SAVR).8 This was also seen in the PARTNER 3 trial where procedure time for TAVI was 59 minutes compared to 208 minutes, thereby contributing to the overall cost-saving6.
TAVI using the SAPIEN 3 valve was also associated with a >50% reduction in total rehabilitation/skilled nursing days (versus SAVR) and a cost-saving of $5,169 in low-risk patients in the US.3 In an Italian cohort of intermediate patients, rehabilitation costs following TAVI using SAPIEN 3 valve were €1,594 less than SAVR.1
Despite considerable cost saving benefits of TAVI compared to SAVR, there are ways to optimise this further. One way is the use of M-TAVI, which entails moderate sedation, percutaneous vascular access, and post-implant transthoracic echocardiography (TTE); M-TAVI has similar rates of complications to conventional TAVI, as well as lower all-cause mortality.5,9
Compared to conventional TAVI, length of stay associated with M-TAVI was 28% shorter and patients were more likely to be discharged home.9 The overall length of hospital stay for multidisciplinary, modality, minimalist (3M) TAVI more than half that of conventional TAVI using the SAPIEN 3 valve (1.6 vs. 3.9 days) incurring an in-hospital cost-saving of $4,377.10 In a UK-based study, mean ICU length of stay was 5.1 hours for M-TAVI and 57.2 hours for conventional TAVI, incurring a saving of £1,638.5
The shorter total procedure time of 115 minutes, compared to 181 minutes for conventional TAVI using the SAPIEN 3 valve incurred a cost saving of $1,551.10 Drug costs were also £213 less in the M-TAVI group, giving an overall cost saving of £3,580 despite the initial valve costs being considerably higher.5
For professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable). Edwards devices placed on the European market meeting the essential requirements referred to in Article 3 of the Medical Device Directive 93/42/EEC bear the CE marking of conformity.
Edwards, Edwards Lifesciences, PARTNER, PARTNER 3, SAPIEN, and SAPIEN 3 are trademarks or service marks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
© 2022 Edwards Lifesciences Corporation. All rights reserved. PP–EU-4528 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
11th July 2022
Many studies have shown improved efficacy of transcatheter aortic valve implantation (TAVI) compared with surgical aortic valve replacement (SAVR) in improving clinical symptoms and quality of life in patients across all-risk aortic stenosis.1,2,3 It is well-documented that, compared with SAVR, TAVI reduces length of stay and rehospitalisation, lowers stroke risk, and reduces all-cause mortality.1
Less well-documented is the cost effectiveness of TAVI compared with SAVR. Despite the adoption of minimalist TAVI,4,5 improved technology and increased eligible patient populations, the cost effectiveness of TAVI remains a highly contentious issue.6
It is reported that there are 115,000 eligible aortic stenosis candidates for TAVI who are deemed inoperable or of high and intermediate risk across the EU; this is expected to increase to 177,000 with expansion into low-risk patients.7 Given this rapid growth in TAVI uptake, there is a great need for economic evaluation; particularly, in relation to allocation of scarce healthcare resources.7,8
This has prompted several studies to evaluate the cost effectiveness of TAVI vs SAVR, and recent analyses are consistently showing that TAVI is a cost-effective therapy.6,9-11,13-16 Minimalist TAVI is proven to have increased benefits compared with conventional TAVI and, therefore, a reduction in overall resource utilisation, which is expected to increase the cost effectiveness of TAVI compared with SAVR.12
The economic value of TAVI is confirmed in a study showing that cost savings are driven by lower initial procedural costs and long-term costs with SAPIEN 3 valve compared to SAVR.13 SAPIEN 3 valve also gains additional quality adjusted life years (QALY) through lower risk of mortality and better health-related quality of life.13
These results are consistent with non-European studies. In Australia, the marginally higher procedural costs of TAVI, compared to SAVR, were seen to be offset by shorter lengths of hospitalisation and lower acute complication costs.14 Over the patient lifetime, it was confirmed that balloon-expandable TAVI (BE-TAVI) is cost-effective compared to SAVR, with self-expanding TAVI (SE-TAVI) being the economically dominant option.14 A Canadian study also found incremental cost effectiveness ratios (ICERs) for BE-TAVI and SE-TAVI were $27,196/QALY and $59,641/QALY, respectively.15
The cost effectiveness of TAVI was also reflected in a US cohort who received TAVI using the
SAPIEN 3 valve.16 The PARTNER 3 economic study showed that reduced hospital length of stay was the main factor in offsetting procedural costs and, at a 2-year follow-up, TAVI costs were substantially lower than for SAVR.16 The highest saving was for patients with moderate to severe symptoms, for whom the 2-year cost savings were >$6000/patient.16 In this way, TAVI was confirmed to be the economically dominant strategy with 95% probability of being cost-effective compared to SAVR.16
In high- and intermediate-risk patients with aortic stenosis, TAVI generated higher procedural costs compared with SAVR, which were attributed to the higher valve acquisition costs; however, transfemoral TAVI resulted in greater reductions in length of stay compared with SAVR, which is believed to offset the higher procedural costs, and thus results in a better ICER.8
It has been purported that SAPIEN 3 valve facilitates the efficient management of severe aortic stenosis in high- and intermediate-risk patients, resulting in the cost effectiveness of TAVI.9 Pinar et al. reported cost-effectiveness of TAVI using a SAPIEN 3 valve in high- and intermediate-risk patients in a Spanish economic model, reporting a 75% predictability of cost effectiveness (Table 1).9 Lorenzoni et al. reported cost effectiveness in an Italian economic model for high and intermediate risk (Table 1).10 In both models, it is assumed that TAVI would be cost effective at frequently cited willingness-to-pay thresholds.9,10
Furthermore, Goodall et al. reported the cost effectiveness of TAVI in a French economic model in intermediate-risk patients with the SAPIEN 3 valve (Table 1).11 Reported life expectancy and QALYs (0.42 years and 0.41 QALYs, respectively) demonstrated clear clinical benefits. Lifetime cost savings of €439 with TAVI were reported compared with SAVR, with 100% likelihood of cost effectiveness at a €15,000 willingness-to-pay threshold.11
| Author | Year | Country | Surgical risk | ICER | WTP threshold | Probability of cost-effectiveness | |
|---|---|---|---|---|---|---|---|
| Pinar9 | 2021 | Spain | High | €5329/LYG | 75% | Cost-effective | |
| Intermediate | €7910/LYG | ||||||
| Lorenzoni10 | 2021 | Italy | High | €11,209/QALY | €25,000 | 90-100% | Cost-effective |
| intermediate | €8338/QALY | ||||||
| Goodall11 | 2018 | France | Intermediate risk | €15000 | 100 | Dominant | |
Therapy costs associated with TAVI include the device, procedural costs, initial and repeat hospitalisations, drugs used, perioperative and long-term complications, and physicians’ fees.8 Higher acquisition costs for TAVI compared with SAVR are partially offset in all-risk groups because of its effectiveness and safety profile;10 predominantly, this includes lower procedural costs, shorter length of stay in hospital, and a reduced need for cardiac rehabilitation.11 Therefore, the cost of therapy for aortic stenosis in all-risk surgical populations is not simply a cost comparison of implantable devices, as this ignores multiple factors such as lifetime costs, clinical benefits, impact on hospital resources, capacities and patient preference. 6,8,11 Furthermore, TAVI must result in both improved quality of life and gains in life expectancy in order to be considered cost effective.8
TAVI may become the reference treatment for selected patients with severe AS.13
A comprehensive understanding of the clinical and economic implications of TAVI is necessary to enable appropriate policy and funding decisions; it is expected that further breakthroughs in TAVI technology will heighten its performance to equal or exceed that of SAVR.6,11 As the technique improves, learning curves, economies of scale, and technological innovations may have profound effects on the results.8,17 It is expected that TAVI might be more cost effective when new-generation devices are used and if profound clinical experience is guaranteed.17
For professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards devices placed on the European market meeting the essential requirements referred to in Article 3 of the Medical Device Directive 93/42/EEC bear the CE marking of conformity.
Edwards, Edwards Lifesciences, the stylized E logo, PARTNER, PARTNER 3, SAPIEN, and SAPIEN 3 are trademarks or service marks of Edwards Lifesciences Corporation or its affiliates.
All other trademarks are the property of their respective owners.
© 2022 Edwards Lifesciences Corporation. All rights reserved. PP–EU-4404 v1.0
21st December 2021
22nd October 2021
In August, new guidelines were released as part of the ESC Congress 2021 for the management of valvular heart disease. Included in these guidelines are updated recommendations for the treatment of patients with aortic stenosis.
Updates to treatment recommendations1
Patient eligibility
ESC Guidelines have adopted an age-based approach over a risk-based approach for patients who are
>75 years of age.1 For these patients, transfemoral (TF)-TAVI is now considered the standard of care, irrespective of surgical risk score.1 Furthermore, TF-TAVI is also recommended for patients who are <75 years of age and considered to be of increased risk or inoperable for surgery.1
Empirical evidence suggests that the average age of patients undergoing TAVI remains 80 years of age,2 and it is expected that these new guidelines will increase the referral of patients who are
>75 years of age for TAVI, likely reducing the mean age of patients undergoing the procedure and increasing the TAVI population.
Pivotal role of the Heart Team
In the updated guidelines, more weight is given to the collaborative role of the Heart Team, placing it at the centre of every treatment decision for patients with aortic stenosis.1 The updated guidelines recommend that the choice between surgical or transcatheter intervention must be carefully evaluated by the Heart Team factoring in clinical, anatomical and procedural risks for every patient.1
The Heart Team fosters collaboration across medical specialities to offer optimal patient-centred care and has been shown to improve outcomes in TAVI procedures.3 Alongside making treatment decisions, the Heart Team guides patient selection, optimises patients’ pre-procedure status, facilitates early discharge and provides continuity of care.4,5
Patients’ choice is paramount
Once a decision has been made by the Heart Team, it is now recommended that this is discussed with the patient, allowing the patient to make an informed treatment choice.1
This will renew the focus on incorporating patient values and preferences in a shared decision-making approach.6 Patient-centred goals may inform selection of treatment options aligned with patient preferences. The most reported patient-defined goals in valvular disease are ones that will lead to a better quality of life. In elderly populations with ssAS, patient treatment goals favour quality of life outcomes over survival, and patient-defined goals include maintaining independence, reducing symptoms, and increasing functional ability – all of which favour TAVI over SAVR.7,8
Asymptomatic aortic stenosis
New to this version of the guidelines is recommendations for intervention for asymptomatic patients. Intervention is recommended in case of LV dysfunction or with symptoms on exercise testing.1
Historically, the treatment approach for patients with asymptomatic aortic stenosis has been one of close observation until symptom development.9 Implicit in this approach is the concept that asymptomatic patients have a good prognosis and will not benefit from intervention – instead exposing them to undue risks by undergoing intervention.9 However, more recently, it has been evidenced that long-term prognosis of these patients may not be as favourable as once thought.9 Therefore, the balance of risk vs prognostic benefit has shifted with the belief that some patients who are asymptomatic may benefit from early intervention for prognostic reasons.9
Increase in TAVI eligibility
It is expected that the uptake of TAVI across the EU is expected to increase to as much as 177,000 with major implications for healthcare resource planning.10 These updated guidelines are anticipated to increase the patient population considered for, or undergoing, TAVI.
In Europe, one million people >75 years of age have aortic stenosis, a disease burden that is increasing with an ageing population.11 Currently, only two-thirds of those with AS receive an intervention11 – and it is hoped that these updated guidelines make the referral for TAVI clear to understand, allowing more patients to undergo intervention as well as the possibility that asymptomatic patients with aortic stenosis can now be considered for early intervention.
Maximising capacity and reducing waiting lists by adapting to a growing TAVI need
Demand for TAVI may already exceed the capacity of service provision, and patients face increased waiting list times – which, translate to increased patient mortality.12 A delay of up to 6 months could lead to 24% of patients dying on the waiting list.12
Advances in technology as well as minimalist practices have led to the TAVI procedure being streamlined.12 Many studies have consolidated best practices to develop, implement and evaluate a standardised clinical pathway to facilitate safe discharge home at the earliest time after TAVI.13-15
Benefits of TAVI
Consolidating TAVI organisational efficiencies, it has been demonstrated that a minimalist, streamlined TAVI pathway, with rapid remobilisation, allows for next-day discharge home, with reproducible, excellent safety and efficiency outcomes.Next-day discharge and 48h discharge was achieved in 80% and 90% of TAVI patients, respectively, and amid concerns that a minimalist approach may affect safety or clinical efficacy, the composite primary endpoint of all-cause mortality or stroke by 30 days occurred in 2.9% of TAVI patients.14
For professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards devices placed on the European market meeting the essential requirements referred to in Article 3 of the Medical Device Directive 93/42/EEC bear the CE marking of conformity.
©2021 Edwards Lifesciences Corporation. All rights reserved. PP–EU-2599 v1.0
10th August 2021
COVID‐19 has had an unprecedented impact on the management of patients with aortic valve disease since non-urgent cardiovascular diagnostics and interventions were deferred.1–3 Delays in treatment are leading to a growing number of patients waiting for treatment with longer wait times.2,4 The consequences of such delays include death within the perioperative period and poor operative outcomes.1,4–7 It is estimated that as much as 50% of cancelled or delayed procedures may result in significant harm;6 therefore, delays in elective procedures to treat severe symptomatic aortic stenosis (ssAS) contribute to a hidden mortality rate of COVID-19.8 This is creating a wave of pressure on healthcare resources and personnel;4 reconsideration of these patients for transcatheter aortic valve implantation (TAVI) may help to prevent such complications as well as offering patient and organisational benefits1
COVID-19 has had a significant impact on therapeutic options for ssAS patients undergoing aortic valve replacement (AVR).3
During the pandemic, hospitals have initiated protocols that favour management options that minimise the use of (a) anaesthesiologists, (b) ventilators, (c) operating rooms, and (d) intensive care unit beds. While surgical AVR (SAVR) require all these elements, TAVI does not1,6—using widely described ‘minimalist’ methodology.6,9–11 Guidelines for ssAS patients during the COVID-19 pandemic have, therefore, included the principle that patients who may have been accepted for sAVR could be ‘diverted’ to TAVI under the guidance of the Heart Team.1,2 SAVR has been the treatment choice in AS for decades, nevertheless, TAVI and especially TAVI with transfemoral access, has become a reliable and effective treatment option.1 This has formed a cornerstone of recommendations for TAVI practice during COVID-19.12
The adoption of a minimalist TAVI approach as the preferred default strategy is an imperative to promote access to care in the ‘new normal’ as COVID-19 continues to dictate the priorities of care.4 Perek et al. report that, from their hospital experience, in the years preceding the pandemic (2018 and 2019), approximately 50% of patients underwent SAVR; this rate dropped to 34% during COVID-19 (2020), demonstrating a shift in procedure from SAVR to minimally invasive TAVI.3 Alongside this shift, Joseph et al. report that there was a significant increase in the proportion of SAPIEN 3™ valves inserted (34 vs 68%, p = 0.001).12 During 2020, patients with AS were younger and had a lower calcification burden compared with pre-pandemic practice, making these patients more suitable for the SAPIEN 3™ valve, accounting for the increase in the use of the SAPIEN 3™ valve.12
COVID-19 cardiology guidelines were based on the accumulation of scientific evidence from clinical trials evidencing that minimally invasive TAVI is a safe and efficacious procedure with low complication rates, shorter length of hospital stay, reduced mortality and minimal stroke rate at 30 days, compared with SAVR.3,9–11 TAVI enables patients to rapidly derive significantly improved quantity and quality of life, regardless of surgical risk profile.2,4 From the patient’s standpoint, TAVI is preferable to SAVR, given shorter hospitalisation and consequent exposure of patients to COVID-19 in hospital and rehabilitation centers.8 This is also true from an organisational viewpoint, undoubtedly conserving resources relative to SAVR.8
Joseph et al. report that TAVI can be undertaken safely during the COVID-19 pandemic with 30-day event rates similar with published clinical trials and international registries.12 No statistically significant difference was noted in peri-procedural complications and 30-day outcomes, while post-operative length of stay was significantly reduced (2 vs 3 days, p < 0.0001) when compared with pre-COVID-19 practice.12
The rapid onset of halting referrals and procedures to create capacity to manage the COVID-19 pandemic will be followed by the resumption of access to care under drastically different circumstances, as the world emerges from the pandemic into a ‘new normal’.4 Addressing the escalating needs of patients with cardiovascular disease who are awaiting treatment presents the next challenge for healthcare systems across regions.4 Along with an understanding of the dynamic constraints on healthcare systems, minimalist TAVI can potentially help to further reduce post-care utilisation of resources and allow early patient recovery at home.5
The COVID-19 pandemic has acted as a catalyst for change in healthcare systems worldwide. Resulting adaptations ought to be perceived as opportunities for sustained change and not as temporary disruptions to an often empirically derived TAVI service framework.12 For patients with severe aortic stenosis, efforts to bring treatment to patients amid this pandemic might lead to favoured use of catheter-based management using minimalist techniques.6 As the pandemic abates, TAVI programmes cannot expect a ‘flipping of the switch’ back to pre-pandemic status.4 TAVI programmes must facilitate access to care without compromising patient safety, enable hospitals to manage the competing demands created by COVID-19 and establish new processes to support patients living with valvular heart disease.4
Cost remains a significant barrier to the widespread adoption of TAVI by publicly-funded services outside the pandemic setting; however, incorporating minimalist TAVI has the potential to further improve the cost-effectiveness of a TAVI service.12 There is a compelling need to facilitate the rapid adoption of best practices adapted to the unique demands created by COVID-19 and leverage existing evidence to minimise healthcare resources, facilitate accelerated treatment of AS without compromising patient safety and ensure that patients return home to enjoy the benefits that TAVI affords.4
For professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards devices placed on the European market meeting the essential requirements referred to in Article 3 of the Medical Device Directive 93/42/EEC bear the CE marking of conformity.
©2021 Edwards Lifesciences Corporation. All rights reserved. PP–EU-2599 v1.0