This website is intended for healthcare professionals only.
Take a look at a selection of our recent media coverage:
7th July 2021
This year, the annual meeting of EuroPCR was held as a 3-day digital event, supplemented with on-demand content. EuroPCR historically covers a diverse range of topics in interventional cardiovascular medicine.
There were many sessions dedicated to the evolving changes of transcatheter aortic valve implantation (TAVI). This third and final part of the report details the conference highlights on benefits of the Edwards Benchmark™ program, including improved patient outcomes, organisational efficiencies, and program implementation.
Discussions on optimising TAVI procedures, leading to patient and organisational benefits and resource conservation were perceived as a contemporary concern as the world emerges from the pandemic into a ‘new normal’ post-COVID-19.
| Session type | Session title | Speakers |
|---|---|---|
| Livestream | TAVI: contemporary indications and techniques | Didier Tchetche, Hendrik Treede, Lars Sondergaard & Helene Eltchaninoff |
| Livestream | Optimise your TAVI patient’s outcomes: implementing the Edwards Benchmark program |
Simon Kennon, Olivier Darremont, Helene Eltchaninoff, Derk Frank & Sandra Lauck Francesco Saia |
| On-demand | Improving patient outcomes with Edwards Benchmark program |
Helene Eltchaninoff |
The Edwards Benchmark™ program was introduced by Prof. Helene Eltchaninoff, Dr Sandra Lauck, Dr Olivier Darremont and Dr Francesco Saia; it was described as a multidisciplinary team-based and patient-centred quality improvement program that revisits historical practice and matches contemporary patient care to current TAVI techniques that are based on up-to-date empirical evidence.
An effective, evidence-based program to improve communication and to improve patient’s trajectory of care
Sandra Lauck
The research basis for the Edwards Benchmark™ program focuses on the latest empirical evidence for minimalist TAVI
pathways.1-3 Looking at best practice across the patient trajectory is essentially the spirit of the Benchmark™ program: standardised program processes with increased efficiency, resulting in a minimalist pathway with maximum safety and improved patient outcomes.
When polled on what they believed the benefit of an optimised TAVI pathway to be, 70% of the EuroPCR attendees selected ‘superior patient outcomes’, and 53% selected ‘continuous access to care’. With only 3% selecting ‘unclear benefits’, it is apparent that most of the audience believed that there is some benefit derived from optimising the TAVI procedure pathway – both Dr Francesco Saia and Dr Derk Frank agreed with the audience, commenting that it is great to see that most of the attendees perceive some benefit to optimising the TAVI procedure pathway. In Kiel, Dr Derk Frank is managing to achieve some great results in regard to the mean length of stay, since this has been reduced from 10 to 5 days through standardisation of the TAVI procedure.
Additionally, Dr Sandra Lauck described improved quality-of-life of TAVI was demonstrated from the 3M TAVI study, which took place from 2014 to 2017.1 With most patients deriving significant quality-of-life benefits by Week 2, this offered reassurance that next-day discharge home was well tolerated.
In Vancouver, Dr Sandra Lauck and team are achieving excellent procedural outcomes, 1 which are rounded to form three objectives of the Edwards Benchmark™ program,
with the other three coming from the FAST-TAVI trial: 3
Current variability in hospital length of stay is an opportunity to apply measures that will allow for quicker discharge. Early discharge decreases the overall cost of hospital stay/patient. The Edwards Benchmark™ program provides opportunities to implement measures for optimisation and resource conservation pre-, peri- and post-procedure. This can include shortening and simplifying each of the procedure steps and using protocols to avoid delays in discharge.
In Germany, we recognised the need for a streamlined TAVI program with timely and safe discharge
Derk Frank
When polled, 43% of the audience identified ‘administration buy-in’ as the biggest barrier to implementing a TAVI efficiency program, such as the Edwards Benchmark™ program. This was closely followed by ‘additional capacity’, ‘TAVI coordinator’ and ‘discharge destination’, all ≥30%.
Prof. Helene Eltchaninoff was intrigued by these results since hospital reimbursement for TAVI is based on patients’ length of stay. She recognises that there is a loss of reimbursement with early discharge, but there are stronger medical reasons to discharge early. Reduced hospitalisation equates to increased TAVI procedures, and Dr Francesco Saia added that in the wake of the pandemic, there is a strong need to increase optimisation, organisational efficiencies, and the number of procedures to reduce waiting lists and a backlog of care. Reimbursement, however, may be an issue in other organisations.
Facilitator Simon Kennon added, that in the UK, he does not believe administration buy-in to be a problem, but rather healthcare professional buy-in. He was very interested how this reflects multi-regional differences in healthcare systems.
Loss of reimbursement is trivial compared with increased capacity
Helene Elchaninoff
Dr Derk Frank had the opportunity to present the Edwards Benchmark™ Registry: an ongoing study to reduce hospital length of stay in patients undergoing TAVI and reducing the need for ICU resources. This study is taking place over 30 sites with the aim to recruit 2400 patients, 1500 of which will be prospective.
It is very apparent that TAVI has come a long way since the first successful attempt in 2002.1 As EuroPCR 2021 draws to a close for another year, it is evident where we might expect the research and evolution of TAVI to continue in 2021: honing the patient selection criteria for TAVI to, as Dr Sandra Lauck described, get it right for every patient, first time.
Perhaps more importantly, as the world emerges from the pandemic into a ‘new normal’, there will be an expectation of improved organisational efficiencies to get through a backlog of care: TAVI is a procedure that is minimally invasive and whose pathway has been optimised and streamlined to deliver excellent patient outcomes and confer organisational benefits.
©2021 Edwards Lifesciences Corporation. All rights reserved. PP–EU-2511 v1.0
This year, the annual meeting of EuroPCR was held as a 3-day digital event, supplemented with on-demand content. EuroPCR historically covers a diverse range of topics in interventional cardiovascular medicine.
There were many sessions dedicated to the evolving changes of transcatheter aortic valve implantation (TAVI). This second part of the report details conference highlights on optimising the TAVI procedure with a focus on pathway optimisation, early discharge, and the importance of the TAVI coordinator.
| Session type | Session title | Speakers |
|---|---|---|
| Livestream | TAVI: contemporary indications and techniques | Didier Tchetche, Hendrik Treede, Lars Sondergaard & Helene Eltchaninoff |
| On-demand | Re-framing optimal implantation of the SAPIEN 3 valve in TAVI |
Jonathan Mailey |
| Poster: POS341 | Predictors of early discharge after TAVR | Marco Angelillis, et al. |
Dr Derk Frank, Dr Francesco Saia, and Dr Sandra Lauck outlined why optimising the TAVI procedure is needed, including new challenges presented with COVID-19, increased TAVI workload with an all-risk indication, and untreated patients. As the world emerges from the pandemic, optimising resources and increasing hospital capacity will become the biggest challenges according to Dr Francesco Saia. While Dr Sandra Lauck believes that the goals in contemporary TAVI should include a predictable patient trajectory of care, excellent patient outcomes and efficient and scalable processes of care.
Expectation in 2021: Getting it right, for every patient, at every touch point, every time
Sandra Lauck
| Since the first TAVI procedure in 2002,1 it has been simplified, and contemporary TAVI is based on a minimalist pathway with maximum safety, leading to improved patient outcomes.2-4 With TAVI expanding into the low-risk patient cohort, the population available to undergo TAVI is growing. For many organisations, this will require optimisation of the procedure pathway to accommodate the increasing number needed to be treated. There is a need to streamline patient management to treat more patients without compromising patient safety. In Dr Francesco Saia’s experience, protocols help to prepare the patient before they arrive at the hospital. These protocols allow for safe transfer to the ward and a continuity of care. The use of protocols allows for standardisation and there exists protocols for every step along the patient’s trajectory of care: from entry to discharge. Deviation from the pathway should be limited and discussed with the heart team. |
Minimalist TAVI is maximal planning
Francesco Saia
Dr Sandra Lauck, Dr Francesco Saia and Dr Olivier Darremont discussed how early discharge contributes to optimisation of the TAVI procedure, conserving resources and delivering improved patient outcomes. With an expected backlog of care and delays to treatment as the pandemic abates, reducing the length of stay is mandatory to increase capacity and allow the possibility of treating more patients. Fast-track 24h protocols make use of risk criteria for early discharge to limit unexpected complications. Early mobilisation post-procedure contributes to early discharge, and the MobiTAVI5 trial has demonstrated the feasibility, safety and efficacy of early discharge, with early mobilisation, conferring additional organisational and patient benefits.
Dr Marco Angelillis, et al. presented a poster on the predictors of early discharge in TAVI. Patients were categorised as either fast track (<3 days) or slow track (>3 days) based on length of stay. Patients whose length of stay was >3 days were analysed for possible predictors of increased length of stay, including procedural complications or clinical and electrographic characteristics. New onset or worsening of conduction disturbances and major or life-threatening bleeding were independently associated with increased length of stay (>3 days). Therefore, it was concluded that only patients with bleeding complications or major conduction disturbance should be monitored >48h. Early discharge of <3 days did not adversely affect 30-day safety patient outcomes.
With TAVI moving into the low-risk patient indications, empirical evidence shows 80% of patients early discharged (<3 days) to home, and that early discharge is both feasible and safe across all-risk patients; however, the goal should not be day-1 discharge but rather early discharge based on patient status, according to Prof. Helene Eltchaninoff.
The TAVI coordinator has been described as the cornerstone for a successful TAVI program. Sandra Lauck explained how the TAVI program coordinator is responsible for coordination with all relevant parties to achieve improved patient outcomes. Derk Frank expanded that this provides a seamless continuation of care. The program coordinator is pivotal in ensuring that healthcare professionals along the patient’s trajectory are aligned on the objectives of care.
The pivotal responsibilities of a TAVI coordinator include leading the program, facilitating patient-focused processes and improving communication. Dr Derk Frank emphasised the importance of the TAVI coordinator by highlighting how the immediate impact can be measured when their TAVI coordinator is on annual leave: in these instances, the mean length of stay increases by 1 day. He described that it is ideal to have 1 coordinator per 100 TAVI procedures performed/year.
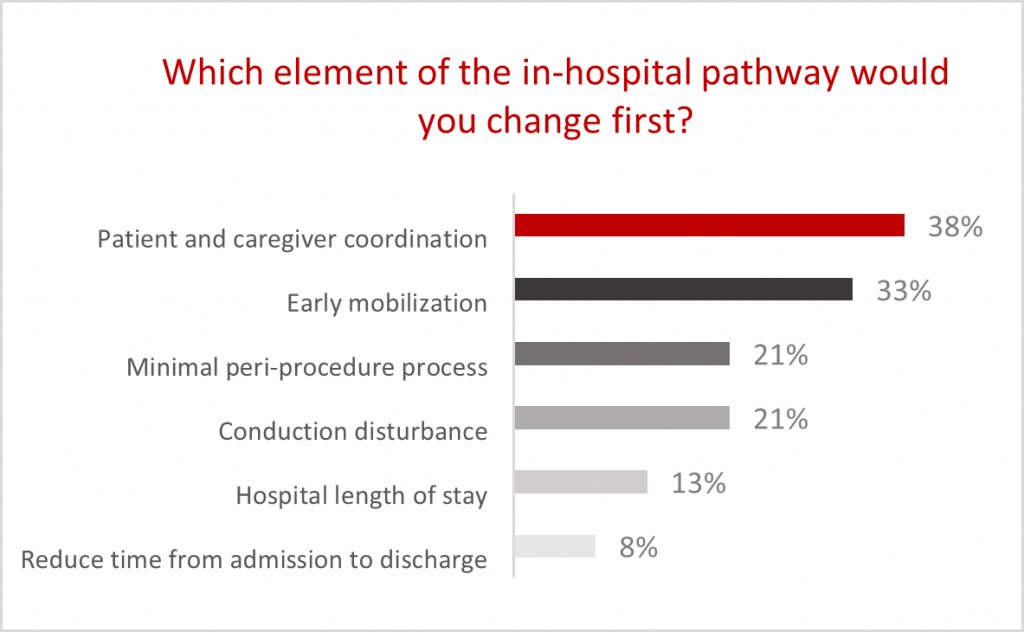
Role of the TAVI coordinator has become an essential indicator of TAVI programs across multiple regions
Sandra Lauck
As the world emerges from the pandemic into a ‘new normal’, there will be an expectation of improved organisational efficiencies to get through a backlog of care: TAVI is a procedure that is minimally invasive and whose pathway has been optimised and streamlined to deliver excellent patient outcomes and confer organisational benefits.
Read the third part of this report here.
1. Cribier A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006–3008.
2. Lauck SB, et al. Vancouver Transcatheter Aortic Valve Replacement Clinical Pathway. Minimalist Approach, Standardised Care, and Discharge Criteria to Reduce Length of Stay. Circ Cardiovasc Qual Outcomes 2016;9:312–21.
3. Wood DA, et al. The Vancouver 3M (Multidisciplinary, Multimodality, But Minimalist) Clinical Pathway Facilitates Safe Next-Day Discharge Home of Low-, Medium-, and High-Volume Transfemoral Transcatheter Aortic Valve Replacement Centres. JACC Cardiovasc Interv 2019;12:459–69.
4. Barbanti M, et al. Optimising patient discharge management after transfemoral transcatheter aortic valve implantation: the multicentre European FAST-TAVI trial. Euro Intervention 2019;15:147–54
5. Vendrik J, et al. Early mobilisation after transfemoral transcatheter aortic valve implantation: results of the MobiTAVI trial. Neth Heart J 2020;28:240-8
For professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable) Edwards devices placed on the European market meeting the essential requirements referred to in Article 3 of the Medical Device Directive 93/42/EEC bear the CE marking of conformity.
[MP1]Added
© 2021 Edwards Lifesciences Corporation. All rights reserved. PP–EU-2467 v1.0
This year, the annual meeting of EuroPCR was held as a 3-day digital event, supplemented with on-demand content. EuroPCR historically covers a diverse range of topics in interventional cardiovascular medicine.
There were many sessions dedicated to the evolving changes of transcatheter aortic valve implantation (TAVI). This first part of the report details conference highlights on the evolving eligible aortic stenosis patient populations for TAVI such as young, low-risk patients and bicuspid aortic stenosis, along with contemporary challenges.
| Session type | Session title | Speakers |
|---|---|---|
| Livestream | TAVI in bicuspid aortic valve | Bernard Prendergast, Simon Redwood, Darren Mylotte & Raj Makkar |
| Livestream | TAVI in bicuspid aortic valve patients, SAPIEN 3™ platform, a safe and efficient option |
Radoslaw Parma, Didier Tchetche, Jörg Kempfert & Christoph Klein |
| Livestream | TAVI: contemporary indications and techniques | Didier Tchetche, Hendrik Treede, Lars Sondergaard & Helene Eltchaninoff |
| On-demand | TAVI for bicuspid aortic valve stenosis in low surgical risk population |
Raj Makkar |
| Poster: POS295 | Five-year results of balloon-expandable TAVI | Olaf Wendler, et al. |
Discussions on appropriate patient selection for TAVI took place with Dr Hendrik Treede, Dr Didier Tchetche, and Prof. Lars Sondergaard. As TAVI is now approved for all-age patients, regardless of the surgical risk profile, categorising patients by surgical risk category may be an outdated process, according to Dr Didier Tchetche. It is far more important to categorise patients based on age, comorbidities, life expectancy, valve anatomy and extent of calcification. Dr Hendrik Treede agreed, adding that, after good results in low-risk trials, even age for TAVI may not be a factor. There is no defined age cut-off for TAVI vs surgical aortic valve replacement (SAVR), but a ‘cut-off’ around 65 years of age might be considered based on life expectancy and valve durability.
The definition of acceptable outcomes has changed based on increased life expectancy
Nicolas Dumonteil
Dr Didier Tchetche explained that most experts agree on a cut-off age of 65 years because of valve durability concerns. With TAVI indications expanding into the low-risk surgical patient population, valve durability becomes one of the biggest contemporary challenges. The younger patient cohort along with increased life expectancy means that patients are expected to outlive their device, and some structural valve deterioration is expected. However, there is a paucity of long-term data on outcomes.
5-year outcomes from the SOURCE 3 registry were reported by Prof. Olaf Wendler, et al. The SOURCE 3 registry included 1947 patients from 80 centres in 10 countries, enrolled between July 2014 and October 2015, who underwent TAVI with the SAPIEN 3™ valve.¹ The results showed that over time, non-cardiovascular causes of deaths increased in the elderly cohort of patients with an infrequent need for re-intervention: only 22 re-interventions were needed. Looking at the 5-year outcomes, it was concluded that the SAPIEN 3 device provides a safe and effective treatment for patients with severe, symptomatic aortic stenosis.
TAVI for bicuspid valve aortic stenosis was a hot topic at this year’s EuroPCR – with the evolution of TAVI into these patients who have previously been excluded from clinical trials based on their anatomy. Bicuspid aortic valve discussions were held with Prof. Bernard Prendergast, Prof. Simon Redwood, Dr Darren Mylotte, Dr Raj Makkar, Dr Didier Tchetche, and Dr Jörg Kempfert.
Bicuspid aortic valve disease is the most common congenital heart disorder in adults, affecting 1-2% of the population, and can be complicated by aortic stenosis. Traditionally, SAVR has been the treatment of choice for symptomatic bicuspid aortic stenosis because of their challenging anatomy: up to 50% of patients undergoing SAVR for AS have bicuspid valves.1 Patients with bicuspid aortic valves are frequently observed in younger patients who undergo SAVR; it has been reported that as many as 41.7% of those who are 70 years of age who undergo SAVR have a bicuspid aortic valve.1 As TAVI expands into the intermediate- and low-risk patient cohorts, this has critical implications for TAVI and valve anatomy.1
In such patients, the heart team conduct a thorough assessment, and the patient’s age, severity and distribution of calcification and surgical risk are key factors in the decision-making process.
It is now believed that TAVI is a reasonable alternative to surgery in carefully selected low-risk bicuspid aortic stenosis patients
Raj Makkar
Dr Raj Makkar presented registry results that examined outcomes of TAVI in bicuspid valvular aortic stenosis.³ 160,000 low-risk patients underwent TAVI with SAPIEN 3™ and SAPIEN 3 Ultra™ valves: of these, 3,243 had bicuspid valves with a mean age of 69 years. Procedural and in-hospital outcomes were reported to be excellent with all-cause mortality reported as 0.6% vs 0.4% in bicuspid and tricuspid valves, respectively. The primary endpoint of stroke was 1.1% vs 0.9% in bicuspid and tricuspid valves, respectively. Despite concerns that surround the use of TAVI in bicuspid anatomy, procedural success rate was high and intraprocedural complications were low. The rates of death and/or stroke at 30 days and 1 year were found to be favourable in low surgical risk patients undergoing TAVI for bicuspid aortic stenosis and similar to tricuspid aortic stenosis. These results suggest that TAVI may be a reasonable treatment option in carefully selected patients with bicuspid aortic stenosis who are of low surgical risk.
It is very apparent that TAVI has come a long way since the first successful attempt in 2002. As EuroPCR 2021 draws to a close for another year, it is evident where we might expect the research and evolution of TAVI to continue in 2021: honing the patient selection criteria for TAVI to, as Dr Sandra Lauck described, get it right for every patient, first time. Long-awaited long-term data on valve durability is highly anticipated and will guide TAVI treatment decisions and finally, following such promising early results, there is an expectation of more research with promising outcomes in performing TAVI in a before-now excluded patient group for TAVI: the bicuspid aortic stenosis patient.
Read the second part of this report here.
For professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable). Edwards devices placed on the European market meeting the essential requirements referred to in Article 3 of the Medical Device Directive 93/42/EEC bear the CE marking of conformity.
© 2021 Edwards Lifesciences Corporation. All rights reserved. PP–EU-2467 v1.0