A research team from the Netherlands has found that the stability of monoclonal antibodies (mAbs) is unaffected by the use of drone transportation when compared with traditional car transport for inter-hospital transportation.
They found the stability of mAbs in both vials and infusion bags was adequately maintained during drone transport, indicating that medical drones are a viable and reliable transportation method.
The current reliance on cars to deliver mAbs is compromised by the unpredictable nature of traffic and infrastructure, which leads to unreliable transport times. Drone transport could therefore offer health systems a more efficient and predictable transportation system for mAbs, the authors said.
Efficacy and safety of drone transportation for mAbs
The researchers assessed the efficacy and safety of medical drone transportation on the stability of mAbs transported in vials and ready-to-administer infusion bags with blinatumomab, tocilizumab and daratumumab. These were Blincyto 38.5 μg concentrate after reconstitution, RoActemra 20 mg/mL concentrate for infusion and Darzalex 20 mg/mL concentrate for infusion, respectively.
These mAbs were chosen as blinatumomab represents a low-concentrate protein drug, tocilizumab represents a drug that may be necessary in emergency settings, and daratumumab is a viable option for home treatment of patients.
Using a VTOL fixed-wing drone, temperature recorders and impact indicators estimated mechanical stress during the flight. Four flights carrying vials and five flights carrying IV bags were performed at a field lab in the Netherlands with an average flight length of eight kilometres and eight minutes of flight time.
Control high-performance size-exclusion chromatography (HP-SEC), dynamic light scattering (DLS), light obscuration (LO), micro-flow imaging (MFI) and nanoparticle tracking analysis (NTA) and absence of visible particles (VI) were employed to assess the presence of aggregates and particle formation in car, drone and control conditions.
Where there was enough quality data, statistical analysis of the vials revealed no significant differences between the control group and the drone-exposed mAbs. Similarly, there were no statistically significant differences between the control, the car and the drone groups for all the infusion bags. By way of example, the data for blinatumomab has been summarised in Table 1 below.
Table 1: Summary of the main results of infusion bag analysis
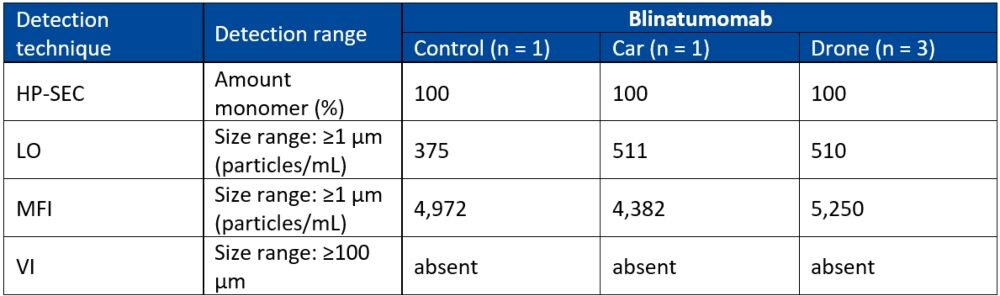
HP-SEC: mean percentage, DLS: mean particle size, LO and MFI: mean total particle concentration, VI: absence of visible particles.
The researchers concluded that integrating drone technology into healthcare logistics has the potential to significantly enhance the efficiency of inter-hospital transportation. Both commercial vials and ready-to-administer infusion bags of mAbs could be transported by drone without resulting in aggregate formation.
Reference
Güngören M et al. Investigating the Impact of Drone Transport on the Stability of Monoclonal Antibodies for Inter-Hospital Transportation. Journal of Pharmaceutical Sciences 2024; Apr 03: doi.org/10.1016/j.xphs.2024.04.002.










