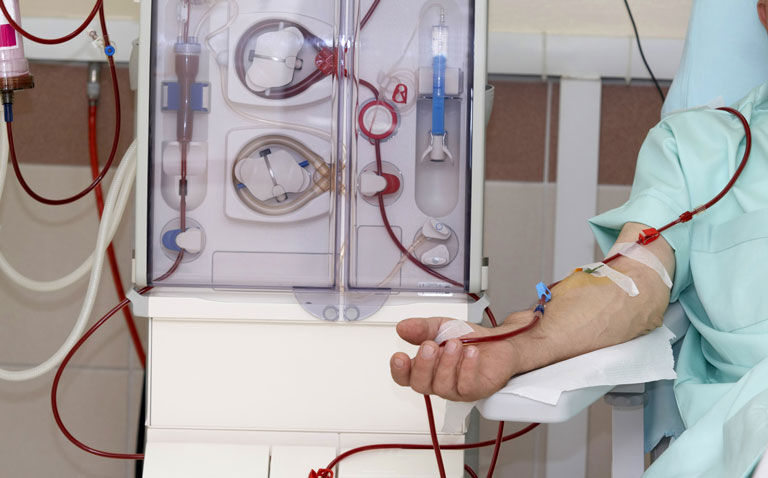
A novel extracorporeal liver device has been found to be safe and able to reduce endotoxaemia and improve albumin function in patients with acute-on-chronic liver failure (ACLF) in a recent randomised trial.
Published in the Journal of Hepatology, researchers demonstrated that the novel extracorporeal liver device, DIALIVE, was as safe as standard care (SC) for patients with ACLF. DIALIVE is designed to achieve removal and replacement of dysfunctional albumin and reduction in endotoxaemia. However, prior to the recent trial, this had not been tested in humans.
ACLF is a syndrome that occurs in hospitalised patients with cirrhosis who present with acute decompensation and carries a high short-term mortality in excess of 15% after 28 days. It is characterised by intense systemic inflammation and associated with multiple organ failures.
Safe and effective for ACLF
Some 32 patients with alcohol-related ACLF were included in the trial and treated with either DIALIVE for up to five days or SC. The study endpoints were assessed at day 10. The primary outcome of interest was safety, defined in terms of the percentage of patients who experienced at least one serious adverse event (SAE) between days one and 10. A number of secondary outcomes were assessed including the change in plasma endotoxin level, albumin function, 28-day mortality and changes in individual organ function.
A total of 17 ACLF patients were randomised to receive DIALIVE and 15 to standard care. The DIALIVE therapy was administered for a median of three sessions and each session lasted for between eight and 12 hours.
The presence of any adverse event was similar in the two groups of ACLF patients (76.5% vs 80%, DIALIVE vs SC, p = 1.00). Similarly, the incidence of serious adverse events in those assigned to the novel extracorporeal device was also not significantly different (p = 0.76). There were also no differences in 28-day mortality.
While there was a trend towards a reduced endotoxaemia severity in the DIALIVE group, this difference was non-significant (p = 0.14). In contrast, there were significant differences favouring DIALIVE for the improvements in liver, kidney, coagulation and brain damage scores. In addition, there was a greater improvement in albumin function in the DIALIVE group. Finally, a higher proportion of patients achieved ACLF resolution with DIALIVE treatment (43% vs 27%).
The authors concluded that DIALIVE achieved its aims of reducing the level of endotoxin and improving albumin function in those with severe liver failure, ultimately leading to a greater proportion of patients whose condition resolved.