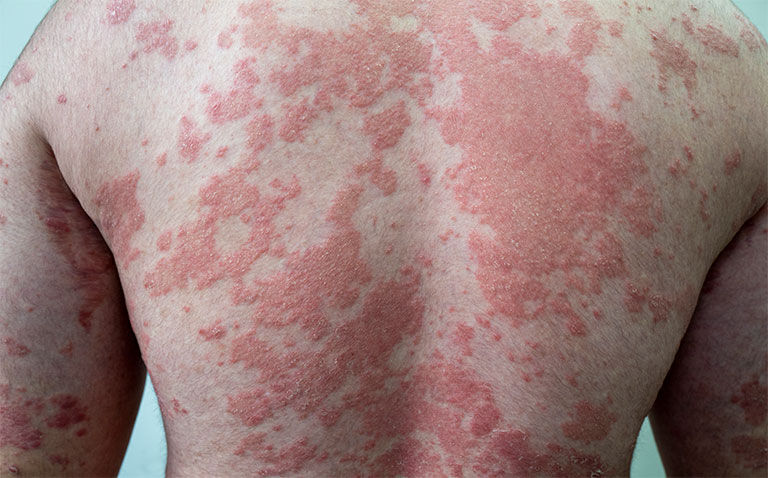
JNJ-77242113 is the first oral peptide able to directly bind to the interleukin-23 (IL-23) receptor, and appears to be effective in moderate to severe plaque psoriasis.
Data presented at the recent 25th World Congress of Dermatology (WCD) from the FRONTIER 1 trial, suggests that JNJ-77242113 (also known as JNJ-2113), a first-in-class oral IL-23 inhibitor, provides a significant improvement in Psoriasis Area Severity Index (PASI) scores compared to a placebo in a dose ranging study.
In the phase 2, randomised, placebo-controlled trial presented at WCD, patients with moderate-to-severe plaque psoriasis were randomised to six different dosing regimens taken either daily (QD) or twice a day (BID) for a total of 16 weeks: 25 mg QD, 50 mg QD, 100 mg QD, or 25 mg BID, 100 mg BID, 100 mg BID or placebo.
The primary outcome was the proportion of patients achieving a 75% improvement in PASI scores at week 16 – known as PASI75. In addition, the team also considered the proportion achieving a PASI90.
JNJ-77242113 and psoriasis outcomes
At week 16, only 9.3% of participants achieved a PASI75. In contrast, a PASI75 was achieved by 37.2% of those given 25 mg and 58.1% of those with a 50 mg daily dose. The highest proportion of patients achieving a PASI75 was for the 100 mg twice daily dose (78.6%).
In addition, while only 2.3% of placebo patients achieved a PASI90, this occurred with 25.6% of those given the 25 mg dose and 59.5% of those given 100 mg twice daily.
For both PASI75 and PASI90 all comparisons with placebo were statistically significant (nominal p < 0.02).
The proportions of patients with adverse events were similar for the different doses of JNJ-77242113 and the placebo group, mainly Covid-19 and nasopharyngitis with no dose-dependent trends.
A recent study found that use of biologics in women with psoriasis who are either pregnant or planning to conceive is not associated with an increased risk of miscarriage, abortion or congenital malformations.