This website is intended for healthcare professionals only.
Take a look at a selection of our recent media coverage:
9th September 2019
6th September 2019
Cardiovascular diseases represent a global burden because of related high morbidity, high mortality and a significant impact on health economies.1 We all play a role in the fight against cardiovascular diseases, and laboratory tests are important to assist clinicians in the prevention, diagnosis and prognosis of cardiovascular diseases. Heart failure (HF) is the inability of the heart to respond to the circulatory demand of the organism. The main causes of HF are hypertension and ischaemic and valvular injuries, whereas toxic, metabolic or genetic origins are less common. In addition to the initial abnormality, secondary changes occur over the course, leading to multi-organ impairment.1,2
More than 20 million people worldwide are estimated to suffer from HF.1,2 HF is increasing because of an ageing population, as a result of the success in prolonging survival in patients having coronary events, and the success in postponing coronary events by effective prevention in those at high risk or those who have already survived a first event. There are several forms of HF (with reduced left ventricular ejection fraction or with preserved left ventricular ejection fraction) challenging both the diagnosis and the risk estimation pathways.1 Understanding HF and its related molecular pathways is fundamental because it allows the identification of potential new biomarkers for patients’ management and potential innovative therapeutic approaches.
Most of the biomarkers with potential applications in diagnosis and prognosis derive from the neurohormonal response to the failing myocardium.2,3 Neurohormonal activation plays a significant role in myocardial and multi-organ adaptations to HF. The use of biomarkers for the diagnosis of suspected HF patients is part of daily procedures and testing for B-type natriuretic peptide (BNP) and the biologically inactive N-terminal fragment (Nt-proBNP) is included in guidelines of scientific societies. Biomarkers may also fulfil complementary information for the evaluation of disease severity, prognosis estimation and for treatment selection.1–3
Natriuretic peptides, the standard of care
Natriuretic peptides are the most recognised and used biomarkers for the diagnosis and monitoring of HF.1-3 The natriuretic peptide family features three members: atrial natriuretic peptide (ANP); BNP; and C-type natriuretic peptide (CNP). BNP is synthesised in the ventricles as a 108- prohormone undergoing a cleavage generating the C-terminal 32 amino-acid active peptide (BNP) and the inactive N-terminal fragment (Nt-proBNP). BNP synthesis and release are essentially stimulated by a ventricular stretch due to pressure or volume overload. Natriuretic peptides are sensitive markers of cardiac dysfunction and useful biomarkers to rule out HF in emergency departments and have also
value for risk stratification of HF because their circulating levels show strong negative correlations with left ventricular ejection fraction and are related to the disease severity (as determined using the NYHA classification).
Many new players are emerging and biomarkers of cardiac remodelling could provide additional information to natriuretic peptides testing and help to develop more tailor-based strategies for treatment.2,3 As previously mentioned, the remodelling and fibrosis of the heart plays an important role in the progression of HF. Biomarkers related to cardiac hypertrophy, cardiac fibrosis and remodeling of the extracellular matrix could provide valuable information for the risk stratification of HF patients. Soluble ST2 and fibroblast growth factor 23 (FGF-23) are two good examples of biomarkers related to remodelling, and automated assays are emerging to facilitate their measurement in clinical laboratories.4–8
Soluble ST2
Interleukin 33 (IL-33) is a member of the IL-1 cytokine family acting both as a cytokine and as an intracellular nuclear factor with transcriptional regulatory properties. IL-33 prevents the apoptosis of cardiomyocytes and improves cardiac function and survival after myocardial infarction through ST2 signalling.4,7 ST2 is a receptor encoded by IL1RL1 and for which differential splicing of the gene can produce a functional membrane-bound receptor (ST2L) or a soluble decoy receptor (sST2) able to quench the biological activity of IL-33. The increase of circulating sST2 levels is related to cardiac remodelling, fibrosis and HF, and measurement of sST2 could facilitate the risk stratification and treatment of HF with reduced ejection fraction as well as the diagnosis and prognosis of HF with preserved ejection fraction.4,7,9
FGF-23
FGF-23, a key regulator of the phosphorus homeostasis, is produced by osteocytes and binds to renal and parathyroid FGF-Klotho receptor heterodimers, resulting in phosphate excretion, decreased 1-a-hydroxylation of 25-hydroxyvitamin D and decreased parathyroid hormone (PTH) secretion.5,6 As for PTH, impaired homeostasis of cations and decreased glomerular filtration rate might contribute to the rise of FGF-23. The amino-terminal portion of FGF-23 (amino acids 1–24) may serve as a signal peptide allowing the secretion into the blood, and the carboxyl-terminal portion (amino acids 180–251) participates in its biological action. FGF-23 is also related to the risk of cardiovascular diseases and mortality.5,6 FGF-23 levels are independently associated with left ventricular mass index and hypertrophy as well as mortality in patients with chronic kidney disease. Increased circulating concentrations of FGF-23 are independently associated with the risk of developing HF in the community and with poor clinical outcome in HF patients, and assays for the measurement of circulating concentrations of the intact hormone (iFGF-23) and some against the C-terminal fragments of FGF-23 (Ct-FGF-23) are available.
Progress around point of care testing (POCT) technologies is enormous, contributing to increased reliabilities and the number of tests available.2,7,10 The added value of POCT is increasingly evident for rapid diagnosis and might add value in primary care and pre-hospital settings. An example is illustrated by the integration of tele-cardiology units and central laboratories through cardiac markers performed with POCT technologies in the ambulance.10 These procedures can play an important role in the early diagnosis and treatment of acute coronary syndrome patients related to the pre-hospital phase. The performances of some POCT assays are now compatible with the enquiries of physicians for the management and monitoring of HF, and both BNP and NT-proBNP can be determined by POCT assays. The implementation of POCT will of course rely on interactions between laboratory specialists and users to respect the requirements of accreditation standards and to maximise the efficiency of POCT-based protocols.7,10
Beside the shift of paradigm for biomarker testing, recent progress in the area of mobile Health (mHealth) is also spectacular.10 MHealth describes the use of portable electronic devices with software applications to provide health services and manage patient information. With approximately five billion mobile phone users globally, mHealth technologies have the potential to greatly impact health research, health care, and health outcomes. Mobile phones, smartphones, and tablets are therefore exceptional means for the empowerment of patients with chronic illness. The use of mHealth technologies decreases the number of disease-related health outcomes in patients suffering from chronic diseases compared with regular care.10 In HF patients, mHealth aid in decreasing the length of stay in hospitals and in maintaining the activities of daily living. Studies involving patients with hypertension also demonstrated the ability of mHealth to reduce systolic and/or diastolic blood pressure.10
Innovations are also coming from the field of data mining and integration, which will allow the combination of clinical and biological features for a more accurate management of patients and will facilitate the identification of clusters of patients at higher risk or more suitable for selection for clinical trials.
To reach maximum potential, innovative biomarkers and emerging technologies will require a multidisciplinary assessment of technical, clinical and economical outcomes, meaning that the communication between specialists in laboratory medicine and other healthcare professionals will be needed to ensure an efficient translation into daily
practice.
References
1 Timmis A et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur Heart J 2018;39(7):508–79.
2 Gruson D, Thys F, Verschuren F. Diagnosing destabilized heart failure in the emergency setting: current and future biomarker tests. Mol Diagn Ther 2011;15(6):327–40.
3 Gruson D, Ko G. Galectins testing: new promises for the diagnosis and risk stratification of chronic diseases? Clin Biochem 2012;45(10-11):719–26.
4 Gruson D et al. Increased soluble ST2 is a stronger predictor of long-term cardiovascular death than natriuretic peptides in heart failure patients with reduced ejection fraction. Int J Cardiol 2014;172(1):e250–2.
5 Gruson D et al. Comparison of fibroblast growth factor 23, soluble ST2 and Galectin-3 for prognostication of cardiovascular death in heart failure patients. Int J Cardiol 2015;189:185–7.
6 Gruson D et al. Head to head comparison of intact and C-terminal fibroblast growth factor 23 in heart failure patients with reduced ejection fraction. Int J Cardiol 2017;248:270–3.
7 Gruson D et al. Testing for soluble ST2 in heart failure patients: Reliability of a point of care method. Clin Lab 2017;63(1):141–5.
8 Lepoutre T et al. Measurement Nt-proBNP circulating concentrations in heart failure patients with a new point-of-care assay. Clin Lab 2013;59(7-8):831–5.
9 Gruson D et al. Increased soluble ST2 is a stronger predictor of long-term cardiovascular death than natriuretic peptides in heart failure patients with reduced ejection fraction. Int J Cardiol 2014;172(1):e250–2.
10 Gruson D, Ko G. Laboratory medicine and mobile health technologies at crossroads: Perspectives for the management of chronic diseases. Crit Rev Clin Lab Sci 2016;53(5):352–7.
The incidence of coronary, peripheral artery (CAD and PAD) and cerebrovascular diseases is rising in the western world, within an ageing population with comorbidities such as diabetes mellitus, renal failure and obesity. Despite advances in pharmacological treatment, many patients with CAD and PAD require invasive treatment to reduce the symptoms of angina or claudication, salvage myocardial or peripheral muscle, and prevent cardiac death or amputation with subsequent disability in those presenting with acute coronary syndromes (ACS) or critical limb ischaemia (CLI), respectively. For both CAD and PAD, endovascular techniques are widely accepted as first-choice treatment options in most patients. This is attributed to significant technological advances over the last decades. However, in patients with severely calcified or/and chronically totally occluded (CTO) coronary or peripheral lesions, antegrade wire passage may be difficult due to the presence of severe calcification in the area of the proximal cap of the occlusion. In addition, even in cases of successful antegrade or retrograde wire passage, standard interventional treatment options, such as balloon angioplasty, drug-coated balloon (DCB) angioplasty and stent placement may fail. In such cases, advanced techniques may become necessary for tackling such complex and calcified lesions. Advanced age, diabetes mellitus and renal disease, especially chronic haemodialysis, have all been associated with increased coronary and peripheral artery calcification, increasing the need for advanced endovascular techniques beyond balloon angioplasty and stent placement in such patients.
Despite modern coronary drug-eluting stent (DES) technologies, calcified coronary lesions remain a great challenge in interventional cardiology. Thus, severe and especially concentric calcification is associated with inadequate balloon expansion and recoil after balloon angioplasty and with failure to deliver a stent or with suboptimal DES expansion, which is a predictor for both periprocedural complications and stent failure in the long-term due to stent thrombosis or in-stent restenosis.1 With the introduction and implementation of adjunctive techniques, such as rotational atherectomy, scoring balloon angioplasty and recently orbital atherectomy and intravascular lithotripsy, the endovascular treatment of such severely calcified lesions has become increasingly feasible, more predictable and therefore safer.
Methods for facilitating crossing of complex lesions, include the use of microcatheters, extension catheters to increase back-up support, over-the-wire balloons, as well as the use of large 7F or 8F support catheters and of anchor balloon support techniques.2
Non-compliant, cutting and scoring balloons
After successful crossing of calcified coronary lesions with a guide wire, the use non-compliant balloons at high pressures is necessary to predilate such lesions. However, lesions with severe concentric calcification may become resistant even to non-compliant balloons despite inflation at high pressures. In addition, the use of high pressures in such stiff calcified lesions may cause dissection or even coronary rupture.3
In cases where non-compliant balloons cannot properly expand, the use of scoring balloons may facilitate better lesion preparation. Such balloons are surrounded by external nitinol spiral scoring wires and are more flexible and better deliverable than previously used cutting balloons. Dilatation using such balloons creates a ‘scoring’, that is, a calcium fracturing’ effect into the calcified and fibrotic tissue of the lesion through a focused transmission force, applied by the very distal portion of these elements. Generally, scoring balloons may be successful for the treatment of moderately to severe calcified coronary lesions.
Rotational atherectomy
Rotational atherectomy has been introduced in the field of interventional cardiology about three decades ago, primarily aiming at mechanical debulking of severely fibrocalcific atherosclerotic plaque. This technique provides ablation of fibrocalcific plaque components through a high-speed rotating burr (~140,000–180,000rpm), whereas non-fibrocalcific, elastic components are spared by deflecting away from the burr. Current guidelines recommend the use of rotational atherectomy for plaque modification before adjacent treatment with balloon angioplasty and DES placement. Although a recent randomised trial was not able to demonstrate a long-term benefit for the use of rotational atherectomy in complex calcified coronary lesions, this technique is widely accepted as the default strategy for such lesions prior to DES placement.4 An example of a severely calcified lesion in the circumflex artery of a patient treated with rotational atherectomy prior to the implantation of three DES is shown in Figure 1.

Figure 1: Images of an 82-year old female patient, with history of 3 vessel CAD and prior bypass surgery, who was referred to our department with non-ST elevation myocardial infarction. Coronary angiography showed a patent left mammary graft to the left ascending coronary artery, a functionally occluded right coronary artery (not shown) and multiple tight lesions in the circumflex coronary artery (blue arrows in A). During prior bypass surgery nine years ago, a graft could not be inserted to the circumflex artery due to severe calcification. We therefore proceeded with rotational atherectomy of the circumflex artery (B–E), which was followed by the implantation of three DES in the circumflex and in the left main coronary artery, with a good final angiographic result in F.
Orbital atherectomy
With orbital atherectomy, an eccentrically mounted diamond-coated crown that orbits 360 degrees within the vessel is used for circumferential plaque removal. Calcific plaque tissue can be removed in this way without causing vessel wall trauma. In contrast to rotational atherectomy, which is limited by the size of the catheter tip or burr size, the debulked area can be increased by increasing the rotational speed of the eccentrically mounted crown. Like rotational atherectomy, orbital atherectomy is also recommended in severely calcified coronary lesions to provide plaque modification prior to balloon angioplasty and DES placement. Orbital atherectomy was approved in 2013 in the US and has approved in Europe in 2018. This technique is available for both coronary and peripheral vessels.
Intracoronary lithotripsy
Intravascular lithotripsy uses pulsatile mechanical energy in order to disrupt calcific lesions, similar to extracorporeal lithotripsy used to disrupt kidney stones. This is facilitated by a balloon angioplasty catheter, containing a series of electrohydraulic lithotripsy emitters, which are used to convert electrical energy to transient acoustic pressure pulses. The single use balloon catheter is connected to a generator, which enables the delivery of prespecified pulse per treatment. First human studies demonstrated the safety and high efficacy of the lithotripsy catheter balloon angioplasty for the treatment of heavily calcified lesions, exhibiting a low rate of major adverse events during 30 days of follow-up.5 In this regard, high resolution optical coherence tomography revealed multiple calcium fractures, enabling area gain for the delivery and expansion of DES as the mechanism of action. This technique is available for both coronary and peripheral vessels.
Due to recent technological advances a minimally invasive endovascular approach is in the meanwhiles widely accepted for the treatment of symptomatic patients with PAD. Commonly used techniques include plain balloon angioplasty, DCB angioplasty, bare metal stents and drug-eluting stents. All these devices have been successfully used to treat claudication symptoms and have achieved limb salvage in CLI patients.6,7
Endovascular approaches, however, may be compromised by severe calcification. Calcification may be the reason for a poor primary outcome due to early recoil or extensive flow-limiting dissections after high-pressure angioplasty.8 Such mechanical effects increase the probability of the need for bailout stent placement, which even with modern dedicated stent devices is associated suboptimal long-term patency, especially in moving vessel zones.9 With the use of percutaneous plaque modification and debulking techniques based on atherectomy however, such calcified lesions can be tackled more easily after removal or fragmentation of atherosclerotic plaque. More homogeneous balloon expansion at lower pressures can be achieved in this way, which reduces barotrauma while facilitating better drug delivery to the vessel wall during DCB angioplasty, and in many cases obviating the need for stent placement. Some of the techniques available for wire passage with CTO lesions, as well as devices available for debulking and lesion preparation in heavily calcified peripheral arteries, are described below.
Similar to coronary CTO, methods for facilitating crossing of peripheral CTO, include the use of support catheters and the puncture of the distal superficial artery, crural or pedal arteries. Such distal puncture techniques may more easily facilitate passage of the occlusive lesion, because like with coronary CTO the distal cap is usually less calcified and therefor easier to penetrate compared to the proximal cap of the occlusive lesion.10
Scoring balloons
Like in coronary arteries, scoring balloons can be used in moderately to heavily calcified peripheral lesions, facilitating improved lesion preparation. In this regard, data from the Heidelberg PANTHER registry indicate that treatment of calcified femoropopliteal lesions with the AngioSculptTM scoring balloon is safe and is associated with a high technical success rate and a primary patency rate of 81.2% at 12 months of follow-up.11
Directional atherectomy
With directional atherectomy, carbide rotating cutter blades are used to cut and remove atherosclerotic tissue. As implied by the name of this technique, the atherectomy device can be guided to the target lesion and rotated in the preferred direction. Thus, directional atherectomy is an optimal technique for the treatment of eccentric lesions. The resected tissue is collected in a nose cone, which must be repeatedly emptied when several passages are necessary. Because no aspiration mechanism is involved, the use of a distal protection filter is mandatory with this device. The safety and efficacy of directional atherectomy has been previously investigated in prospective multicentre studies. One of these studies, the DEFINITIVE AR trial, sought to compare upfront directional atherectomy combined with DCB versus a DCB-only strategy in a randomised setting.12 In this study, combined treatment with atherectomy and DCB was effective and safe; however, no added value was observed in comparison with a DCB-only strategy at one year of follow-up.
Rotational atherectomy
With rotational atherectomy techniques, tissue is concentrically excised using specially designed rotating tips or burrs. The size of the tips or burrs therefore determine the extent of luminal gain. Several systems are available for rotational atherectomy, including the Rotarex®S system (Straub Medical), the Jetstream Atherectomy device (Boston Scientific) and the Phoenix device (Philips Volcano), the latter combining rotational and directional features. The Rotarex®S (Straub Medical, Wangs, Switzerland) consists of an external drive system, connected with the Rotarex®S catheter system. A helix inside the catheter transmits the rotation to the catheter head, which rotates with 10,000 rpm, thus creating a negative suction force. This enables the collection of fragmented tissue into an external bag. Jetstream, on the other hand, is available with two types of catheters, one equipped with a single set of front cutting blades and one with a second set of larger blades to increase the capability of upfront debulking.
This device also provides continuous aspiration and removal of the excised tissue. The Phoenix device is available in sizes of 1.8mm, 2.2mm and 2.4mm. The 2.4-mm catheter possesses a deflecting tip, facilitating a combined directional and rotational atherectomy modus. The Phoenix device possesses a front cutter, which is rotated at high speed (10,000–12,000 rpm), also creating a strong suction force, which allows continuous aspiration of the fragmented plaque components into an external bag. We and others recently demonstrated the safety and efficacy of the Phoenix atherectomy device in femoropopliteal and below-the-knee lesions.13,14 An example of a severely calcified lesion in the common femoral artery of a patient with critical limb ischaemia treated with Phoenix atherectomy, combined with scoring balloon angioplasty and DCB and without stent placement is shown in Figure 2.

Figure 2: Digital subtraction angiography (DSA) images of an 84-year old female patient, with history of severe PAD, who was referred to our department with critical limb ischaemia, Rutherford stage 6. Baseline DSA images demonstrated a tight, heavily calcified lesion of the right common femoral artery (blue arrows in A) and a chronically occluded superficial femoral artery. Phoenix atherectomy was performed in B (blue arrows in B showing bilateral calcifications), combined by AngioSculptTM scoring balloon (C) and Stellarex DCB, with a good angiographic result in D.
With an increasing number of patients suffering from diabetes mellitus and renal failure or haemodialysis, the proportion of patients with complex and calcified coronary and peripheral lesions is already high and is expected to rise even more in the next decades. In addition, with an increasing number of cardiopulmonary and cerebrovascular comorbidities, the proportion of patients at high risk for open cardiac or vascular surgery is also expected to rise in coming years, thus further establishing the role of coronary and peripheral endovascular procedures as the first option for the treatment of such patients. In order to facilitate treatment of such complex fibrocalcific lesions however, advanced treatment options, such as high-pressure and scoring balloon angioplasty together with directional, rotational or orbital atherectomy techniques, have become mandatory and implementation of such techniques will be crucial to improve both acute success and long-term outcomes.
Many FDA-approved atherectomy devices are currently available in the market for coronary and/or for peripheral use. From the practical point of view, such devices are readily widely accepted by interventional cardiologists and angiologists. Considering the availability of diverse scoring balloon and atherectomy devices, it may be advisable to first gain expertise in the use of a single device, optimally applicable for both coronary and peripheral vessels with due attention to patient selection and lesion characteristics.
References
1 Dangas GD et al. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol 2010;56(23):1897–907.
2 Kassimis G et al. How should we treat heavily calcified coronary artery disease in contemporary practice? From atherectomy to intravascular lithotripsy. Cardiovasc Revascularization Med Mol Interv 2019; pii: S1553-8389(19)30040-5.
3 Seth A et al. Expert opinion: Optimising stent deployment in contemporary practice: The role of intracoronary imaging and non-compliant balloons. Interv Cardiol Lond Engl 2017;12(2):81–4.
4 Abdel-Wahab M et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv 2013;6(1):10–19.
5 Ali ZA et al. Optical coherence tomography characterization of coronary lithoplasty for treatment of calcified lesions: First description. JACC Cardiovasc Imaging 2017;10(8):897–906.
6 Fakhry F et al. Endovascular revascularization and supervised exercise for peripheral artery disease and intermittent claudication: A randomized clinical trial. JAMA 2015;314(18):1936–44.
7 Agarwal S, Sud K, Shishehbor MH. Nationwide trends of hospital admission and outcomes among critical limb ischemia patients: From 2003–2011. J Am Coll Cardiol 2016;67(16):1901–13.
8 Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation 1992;86(1):64–70.
9 Cambiaghi T et al. Fracture of a Supera interwoven nitinol stent after treatment of popliteal artery stenosis. J Endovasc Ther Off J Int Soc Endovasc Spec. 2017;24(3):447–9.
10 Giusca S et al. Comparison of ante- vs. retrograde access for the endovascular treatment of long and calcified femoropopliteal occlusive lesions. LINC Congress In Leipzig; 2019.
11 Lugenbiel I et al. Treatment of femoropopliteal lesions with the AngioSculpt scoring balloon – results from the Heidelberg PANTHER registry. VASA Z Gefasskrankheiten 2018;47(1):49–55.
12 Zeller T et al. Directional atherectomy followed by a paclitaxel-coated balloon to inhibit restenosis and maintain vessel patency: Twelve-month results of the DEFINITIVE AR Study. Circ Cardiovasc Interv 2017;10(9): pii: e004848.
13 Davis T et al. Safety and effectiveness of the Phoenix Atherectomy System in lower extremity arteries: Early and midterm outcomes from the prospective multicenter EASE study. Vascular 2017;25(6):563–75.
14 Giusca S et al. Endovascular treatment with the Phoenix Atherectomy System in patients with chronic limb ischemia. A series of seventy-four consecutive patients. LINC Congress In Leipzig; 2019.
In August 2018, the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force published an updated universal definition of myocardial infarction (MI).1
This consensus document provides newly revised criteria for the definition of MI,1 driven by recent advances of modern cardiology. So, what was new in this Fourth Universal Definition of MI and how will this consensus paper affect everyday clinical practice?
Coronary heart disease is one of the leading causes of morbidity and mortality worldwide.2 MI represents the most common, as well as deleterious, form of coronary heart disease, generating a high epidemiological and economic burden of disease.1,2
Considering the high incidence and high global health burden, establishing a general and uniform definition of MI has been attempted over the last decades.1,3 However, different clinical presentations as well as past and ongoing diagnostic advances have given rise to different definitions of MI, leading to controversy and confusion in clinical practice.1
The first general definition of MI dates back to the 1950s, when working groups from the World Health Organization (WHO) established a MI definition mainly on the basis of electrocardiographic (ECG) criteria.1,4
The introduction of cardiac biomarkers with high discriminatory power has particularly revolutionised the detection and diagnosis of MI and necessitated revisions of the global MI definition.1 Modern high-sensitivity cardiac troponin (cTn) assays allow both early detection and fast rule out of MI, which is crucial in daily clinical routine.5,6
By contrast, however, the broad implementation of high-sensitivity troponins into routine laboratory testing has led to a sharply increasing number of incidental findings of elevated troponins without any clinical evidence of ischaemia, frequently leading to clinical misinterpretation.5
The differentiation of such a clinical scenario of myocardial injury without ischaemia from actual MI was a central focus of the newly revised 2018 Universal Definition of MI.1
According to the 2018 consensus document, MI is defined as: (1) acute myocardial injury detected by abnormal cardiac biomarkers; and (2), in a clinical setting with evidence of myocardial ischaemia.1
Abnormality of cardiac biomarkers is defined by a detection of elevated cTn values with at least one value above the 99th percentile upper reference limit (URL).1 If, in addition, a dynamic rise and/or fall of cTn concentrations is detected, the myocardial injury is considered as acute.1
In contrast, a setting of persistently elevated cTn levels without any dynamics would be referred to as chronic myocardial injury.1 Above and beyond acute myocardial injury, evidence of acute myocardial ischaemia is required for the diagnosis of MI.
One or more of the following clinical features affirm acute myocardial ischaemia: typical symptoms of myocardial ischaemia; new ischaemic electrocardiographic changes as well as development of pathological Q waves; imaging evidence of new loss of viable myocardium or new regional wall motion abnormality; and identification of a coronary thrombus by angiography or autopsy.1
Primarily in light of revascularisation strategies, in the early setting of acute coronary syndrome a categorisation into ST-elevation myocardial infarction (STEMI) and non-STEMI is nowadays common clinical practice.1 Beyond these clinical categories used in the early stage, MI can be classified in different types depending on differences in clinical, pathological and prognostic features.1
In the fourth update of the Universal Definition of MI, the five previously proposed types of MI have undergone only slight modifications.1 The criteria of the different MI types are summarised in brief below.
Type 1 MI describes the classical concept of atherothrombotic MI caused by a disruption (rupture or erosion) of an atherosclerotic plaque on the basis of coronary artery disease.1 The resulting thrombus can either be occlusive or non-occlusive.1
The concept of type 2 MI is more complicated and multifactoral, defined by myocardial ischaemia due to a mismatch between oxygen supply and oxygen demand unrelated to coronary atherothrombosis.1 This type of MI remains the most challenging for clinicians because a plethora of different clinical scenarios can lead to type 2 MI.
Importantly, in contrast to coronary atherothrombosis, coronary artery disease per se without plaque disruption does not exclude type 2 MI.1 Indeed, the mismatch between oxygen supply and demand that defines type 2 MI often occurs on the basis of underlying coronary artery disease.1
Potential causes of MI type 2 are acute stressors including severe arrhythmias, severe anaemia, severe hypertension or hypotension, and respiratory failure.1 Such stressors might induce myocardial ischaemia in patients with and without coronary artery disease, whereas individual ischaemic thresholds differ depending on the presence and severity of the underlying coronary artery disease.1
Besides the described mechanistic stressors causing oxygen supply and demand mismatch, coronary pathologies beyond atherothrombosis, such as coronary artery dissection, coronary spasm or coronary embolism, might result in type 2 MI.1
MI type 3 denotes the clinical scenario of cardiac death with symptoms suggestive of myocardial ischaemia and concomitant presumed new ischaemic ECG changes or ventricular fibrillation, but occurrence of death before blood samples for cardiac biomarkers can be obtained.1 Cardiac death with proven MI by autopsy is also referred to as type 3 MI.1
Infarctions in the context of coronary procedures, either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), are defined as type 4 or type 5 MI, respectively.1
Type 4 MI refers to PCI-related infarcts, whereas three subtypes (4a, 4b and 4c) have been suggested in the Fourth Universal Definition of MI.1 Type 4a MI is defined as peri-procedural MI directly related to the index PCI (≤48h after the procedure).1
The criteria of PCI-related MI are arbitrarily defined as elevation of cTn values >5-times the 99th percentile URL in cases of normal baseline values, or increase of >20% in cases of chronically elevated pre-procedural cTn concentrations.1 To allow for definite diagnosis of 4a MI, objective parameters of myocardial ischaemia are required in addition to cTn dynamics.1
Such clinical criteria of myocardial ischaemia are new ischaemic ECG changes and pathological Q waves, imaging evidence of new loss of viable myocardium or new regional wall motion abnormality as well as angiographic findings of procedural flow-limiting complications including coronary dissection, occlusion of an epicardial artery, disruption of collateral flow and distal embolisation.1
Other subtypes of MI associated with PCI are stent or scaffold thrombosis (type 4b MI) and restenosis following PCI (type 4c MI).1 MI type 4b is defined as stent/scaffold thrombosis as detected by angiography or autopsy applying the same formal criteria as used for MI type 1.1
According to the timing of thrombosis occurrence after the index PCI, it should be distinguished between acute (0 to 24 h), subacute (>24 h to 30 days), late (>30 days to 1 year) and very late (>1 year) stent/scaffold thrombosis.1 Type 4c MI describes an in-stent restenosis or restenosis after balloon angioplasty in the infarct-related artery.1
Finally, type 5 MI denotes peri-procedural infarctions in the setting of CABG.1 In contrast to type 4a MI (PCI-related MI), type 5 MI is defined as increase of cTn >10-times the 99th percentile URL in case of normal baseline cTn, or increase of post-procedural cTn >20% in case of chronically elevated pre-procedural cTn-concentration.1
In addition to cTn dynamics, elements of ischaemia including new pathological Q waves, new graft occlusion or native coronary artery occlusion as detected by angiography or loss of viable myocardium or new regional wall motion abnormality by cardiac imaging are necessary for the diagnosis of type 5 MI.1
One of the most important elements of the updated Universal MI Definition is the emphasis on the differentiation between myocardial injury and MI. Only the laboratory finding of elevated cTn or increase of cTn in serial measurements without any clinical evidence of ischaemia should not be labelled as MI but as myocardial injury.1
Moreover, the new consensus paper highlights that myocardial injury should not only be clearly differentiated from MI but also represents an entity in itself, which comprises a variety of diseases requiring further diagnostic workup.
A large number of cardiac (for example, heart failure, myocarditis, Takotsubo syndrome, revascularisation procedure, catheter ablation, defibrillator shocks, cardiac contusion) and non-cardiac (for example, sepsis, chronic kidney disease, stroke or subarachnoid haemorrhage, pulmonary embolism, chemotherapy) pathologies and conditions can lead to myocardial injury unrelated to ischaemia.1
Depending on the presence or absence of a dynamic cTn pattern, the condition of myocardial injury should be declared as acute or chronic.1 The clinical differentiation between the various causes of myocardial injury can be challenging and often requires comprehensive diagnostics.
In this regard, the 2018 Universal Definition of MI has particularly emphasised the use of cardiac magnetic resonance (CMR) imaging to define the aetiology of myocardial injury.1 Indeed, CMR enables a unique in vivo view on myocardial tissues and the use of late gadolinium-enhanced sequences allows for a reliable distinction between ischaemic and non-ischaemic patterns of myocardial scarring.1,7,8
Contingent upon the primary disease, a clear differentiation between myocardial injury and MI might be difficult. As highlighted by a new section in the 2018 consensus document, chronic kidney disease (CKD) especially represents a condition that often results in clinical misinterpretation because a high proportion of patients with CKD displays elevated cTn concentrations.1
Importantly, renal clearance of cTn has only minor effects on serum cTn concentrations9, whereas increased ventricular pressure, microvascular dysfunction, anaemia, hypotension and direct toxic effects of uraemia are considered as main mechanisms explaining the myocardial injury in CKD patients.1,10
Acute volume overload in CKD, for example, may lead to both acute myocardial injury and type 2 MI; a clear differentiation, however, may be challenging and often remains insufficient in clinical routine.1 Not only in CKD patients, but also in a large number of other patients, including patients with multiple morbidities or those who are critically ill, the differentiation between myocardial injury and type 2 MI has remained the key clinical challenge following publication of the 2018 Universal Definition of MI.1
Routine primary PCI in the acute setting of MI has revealed that a considerable proportion of MI patients (approximately 10%) does not show significant coronary artery disease.11,12 This clinical scenario has gained more and more attention over the past few years and is referred to as MI with non-obstructive coronary arteries (MINOCA).13
After publication of a position paper of the ESC working group in 2017,13 MINOCA has now for the first time been included in the Universal Definition of MI.1 As the name implies, MINOCA is defined as MI according to the criteria as described in detail above as well as non-obstructive coronary arteries (no coronary artery stenosis of ≥50%) as displayed by acute angiography.13
MINOCA is a heterogeneous entity with several potential underlying causes that should be elucidated by a comprehensive diagnostic algorithm incorporating additional imaging modalities including CMR.1,13
The Fourth Universal Definition of MI published in 2018 provides updated criteria for the definition of the five types of MI established by the preceding consensus documents, with particular focus on the differentiation between myocardial injury and MI.
The entity of myocardial injury describes a clinical scenario of elevated or increasing cTn concentration without any signs of ischaemia. Particularly due to therapeutic consequences, it is crucial to distinguish myocardial injury from MI, which displays both cTn dynamics and clinical evidence of ischaemia.
1 Thygesen K et al. Fourth Universal definition of myocardial infarction 2018. Circulation 2018;138:e618–e651.
2 Roth GA et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 2017;70:1–25.
3 Thygesen K et al. Universal definition of myocardial infarction. Circulation 2007;116:2634–53.
4 Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation 1979; 59: 607–9.
5 Mair J. High-sensitivity cardiac troponins in everyday clinical practice. World J Cardiol 2014;6:175–82.
6 Twerenbold R et al. Clinical use of high-sensitivity cardiac troponin in patients with suspected myocardial infarction. J Am Coll Cardiol 2017;70: 996–1012.
7 Reinstadler SJ, Thiele H, Eitel I. Risk stratification by cardiac magnetic resonance imaging after ST-elevation myocardial infarction. Curr Opin Cardiol 2015;30:681–9.
8 Motwani M et al. Advances in cardiovascular magnetic resonance in ischaemic heart disease and non-ischaemic cardiomyopathies. Heart 2014;100:1722–33.
9 Friden V et al. Clearance of cardiac troponin T with and without kidney function. Clin Biochem 2017;50:468–74.
10 Januzzi JL Jr et al. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J 2012;33:2265–71.
11 DeWood MA et al. Coronary arteriographic findings soon after non-Q-wave myocardial infarction. N Engl J Med 1986;315:417–23.
12 Gehrie ER et al. Characterization and outcomes of women and men with non-ST-segment elevation myocardial infarction and nonobstructive coronary artery disease: results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) quality improvement initiative. Am Heart J 2009;158:688–94.
13 Agewall S et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 2017;38:143–53.
5th September 2019
Novartis has announced that Luxturna® (voretigene neparvovec) has been recommended for use on the NHS as an option for treating RPE65-mediated inherited retinal dystrophies.
Children and adults born with a mutation in both copies of the RPE65 gene can suffer from a range of symptoms from night blindness (nyctalopia), loss of light sensitivity, loss of peripheral vision to loss of sharpness or clarity of vision, potentially progressing to total blindness. Currently, in the UK, it is estimated that 180 patients have mutations in both copies of the RPE65 gene, with less than 50% diagnosed following a genetic test.
Voretigene neparvovec is injected under the retina and carries a functioning RPE65 gene to act in place of the faulty one. It will be the only treatment available in England and Wales for adults and children with this rare progressive genetic condition.
“We are delighted with today’s decision by NICE to recommend voretigene neparvovec for use in patients with vision loss due to a genetic mutation in both copies of the RPE65 gene” said Haseeb Ahmad, Country President of Novartis UK and Managing Director (UK, Ireland and Nordics) of Novartis Pharmaceuticals. “Through effective collaboration with NICE and NHS England, it has been possible to secure rapid access to the first and only one-time gene therapy for patients living with this condition.”
This is the second time Novartis has worked closely with NICE and NHS England to achieve fast access to a cell and gene therapy. While on average it takes 38 weeks within the Highly Specialised Technologies programme, by working with NHS England and NICE early and constructively this timeline was reduced to 20 weeks – an unprecedented timeframe.
“The patient burden is high for those born with a mutation in both copies of the RPE65 gene. The progressive and debilitating nature of this rare genetic condition places a life-long physical, emotional and financial burden on patients and their families,” said Tina Houlihan, Chief Executive at Retina UK. “NICE’s recommendation of voretigene neparvovec marks a pivotal moment as, for the first time, children and adults born with this condition have a much needed treatment option.”
“The progression of inherited retinal degeneration caused by RPE65 gene mutations leads to blindness, which has a profound effect on the lives of affected patients and their carers” said Robert MacLaren, Professor of Ophthalmology at the University of Oxford and Consultant Ophthalmologist at the Oxford Eye Hospital. “Until now, patients had no other pharmacological treatment options and I am absolutely delighted with the decision by NICE to recommend this one-time gene therapy. As a clinician, I believe the true value of voretigene neparvovec is its potential to improve vision in children and adults, and enabling them to participate fully at school, work and in their private lives.”
The NICE recommendation is based on data from a Phase I clinical trial, its follow-up trial, and the first randomised, controlled Phase III gene therapy trial for an inherited disease. The primary endpoint of the Phase III trial was mean change from baseline to one year in binocular multi-luminance mobility test (MLMT). The difference in mean change in binocular MLMT score between patients treated with voretigene neparvovec (n=21) and the control group (n=10) was 1.6 (95% confidence interval: 0.72-2.41; p=0.001). Differences in binocular MLMT performance were observed in the intervention group at day 30 and were maintained over the remaining follow-up visits throughout the four-year period, compared to no change in the control group.
4th September 2019
The figures given in the present document provide the most updated comparative picture of the situation of healthcare and hospitals, compared to the situation in 2006
For several years, hospitals have been required to act more efficiently and to increase productivity. Increased performance is indeed visible. Yet, healthcare systems are facing conflicting trends: short and long-term impacts of financial and economic restrictions; increasing demand of an ever-expanding and ageing population, which leads to more chronic patients; increasing requests and availability of technological innovations; and new roles, new skills and new responsibilities for the health workforce.
To adapt to this situation, the role of hospitals is evolving further. Most health systems have already moved from a traditional hospital-centric and doctor-centric pattern of care to integrated models in which hospitals work closely with primary care, community care and home-care.
The figures given in this article provide the most up-to-date comparative picture of the situation of healthcare and hospitals, compared with the situation in 2006. The figures aim to increase awareness on what has changed in hospital capacity and, more generally, in secondary care provision within European Union member states, generating questions and stimulating debate, and in this way fostering information exchange and knowledge sharing.
The main source of data and figures is OECD Health Statistics (last update November 2018). Data on health expenditure as a percentage of total general government expenditure and on hospital beds in public or private owned hospitals have been extracted from the Eurostat Database on Economy and Finance (last update March 2019) and on Health (last update July 2018), respectively. All European Union member states belonging to OECD are considered, plus Switzerland and Serbia (as HOPE has observer members in these countries), when data are available. In the text, these are reported as EU. Whenever considered appropriate and/or possible, two groups have been differentiated and compared: EU15, for the countries that joined the EU before 2004 (Austria, Belgium, Denmark, Finland, France, Germany, Greece, Ireland, Italy, Luxembourg, Netherlands, Portugal, Spain, Sweden and United Kingdom) and EU13, for the countries that joined the EU after 2004 (Bulgaria, Cyprus, Czech Republic, Croatia, Estonia, Hungary, Latvia, Lithuania, Malta, Poland, Romania, Slovakia and Slovenia). When averages are reported, they result from our own calculations. The considered trends normally refer to the years 2006–2016. When data on 2016 are not available, or they have not been gathered for a sufficient number of countries, the closest year is considered. Some figures are disputed for not being precise enough but at least they give a good indication of the diversity.
From 2006 to 2016, about 50% of the total current health expenditure expressed in purchasing power parity (PPP$) per capita increased on average in the EU. Inpatient care, out-of-pocket payments and pharmaceutical expenditures grew in the considered years as well.
In EU15, the range of total current health expenditure per capita in 2017 was between 2325 PPP$ in Greece and 6475 PPP$ in Luxembourg, whereas in EU13, this range varied from 1722 PPP$ in Latvia to 2775 PPP$ in Slovenia. In Switzerland, this indicator reached 8009 PPP$. Compared with 2006, the total health expenditure per capita in 2016 varied positively in all the countries taken into consideration in this analysis, except in Greece, where the decrease was –12%. Major increases have been registered in EU13: Estonia (+110%), Lithuania (+107%) and Poland (+103%). Smaller increases were registered in Spain (+36%), Italy (+25%) and Portugal (+24%), all belonging to EU15.
Current public health expenditure includes all schemes aimed at ensuring access to basic health care for the whole society, a large part of it, or at least some vulnerable groups. Included are government schemes, compulsory contributory health insurance schemes, and compulsory medical savings accounts. Current private health expenditure includes voluntary health care payments schemes and household out-of-pocket payments. The first component includes all domestic pre-paid health care financing schemes under which the access to health services is at the discretion of private individuals. The second component corresponds to direct payments for health care goods and services from the household primary income or savings: the payment is made by the user at the time of the purchase of goods or use of service.
In 2017, the percentage of public sector health expenditure to the total current health expenditure was higher than 70% in most countries, except for Latvia (55%), Greece (61%), Portugal (67%), Hungary (67%), Lithuania (67%) and Poland (69%) and, outside the EU, in Switzerland (63%).
In the last years, public sector health expenditure accounted on average for 74% of total health expenditure and 15% of the total government expenditure.
Between 2006 and 2016, the public health expenditure in PPP$ per capita increased on average to almost +50% in the EU. The countries that registered the most significant increases are Lithuania (+105%), Poland (+105%) and Estonia (+117%), whereas those that registered a minor increase were Luxembourg (+15%), Portugal (+19%) and Italy (+20%). Greece was the only country where this indicator decreased (–15%) (Chart 1).
Chart 2 shows the trend over the past ten years concerning the share of government expenditure in health. It presents the aggregated data concerning the EU, and the figures of the three countries having the highest and the lowest values in the year 2017, Switzerland included.
In 2017, the percentage of government expenditure devoted to health on total health expenditure ranged from 7% in Cyprus to 20% in Ireland. In Switzerland, this indicator was 6.4%.
The trends illustrated in Chart 2 are generally positive between 2007 and 2017 with an average yearly increase of percentage of government outlays devoted to health by 0.08 p.p. Yet, from 2009 to 2010, this way of development slackened off in many countries, the reasons including the effects of the financial crisis or the shift of interest and priorities to other sectors.
Out-of-pocket payments show the direct burden of medical costs that households bear at the time of service use. Out-of-pocket payments play an important role in every health care system.
In 2016, the private contribution to healthcare spending was around 20% in the EU, ranging from 10% in France to 45% in Latvia. The other lowest values were registered in Luxembourg (11%), the Netherlands (11%) and Slovenia (12%), while the other highest values were registered in Hungary (30%), Lithuania (32%) and Greece (30%). It is worth noting that Latvia, Hungary, Lithuania and Greece are among the countries with the lowest current health expenditure on health in PPP$ that year.
Between 2006 and 2016, the household out-of-pocket payments in PPP$ per capita has increased in all the EU countries, except for Greece (–32%) and Luxembourg (–1%). The most relevant increases in EU15 were in the United Kingdom (+143%) and the Netherlands (+75%). In Switzerland, the indicator varied by +67% in the years taken into consideration. In EU13, according to available data, the abovementioned figure registered highest increases in Latvia (+121%), Czech Republic (117%) and Lithuania (+110%). The total household out-of-pocket payments in PPP$ per capita continued to increase, because the demand of healthcare services and, in turn, the total health expenditure did.
Chart 3 illustrates the trend 2006–2016 of both the total current health expenditure per capita and the private households’ out-of-pocket payments on health. These values present a correlation (R² = 0.4622) showing that there is dependence between the two indicators. The chart highlights the fast growth of both expenses in the countries located in the upper right part of the graph corresponding to countries belonging to EU13. In those in the lowest-left section of the graph, the out-of-pocket payments grew more slowly compared with the total current health expenditure.
In the majority of the EU member states, 30–40% of current health expenditure (excluding investments and capital outlays) finances hospital care. The funds allocated to providers of long-term care, ancillary services, ambulatory care, preventive care as well as to retailers and other providers of medical goods are excluded from this computation.
In 2016, current hospital expenditure represented approximately 38% of total current health expenditure, ranging from 29% and 32% in Germany and Latvia, respectively, to 46% and 47% in Italy and Estonia (Chart 4). In all countries, even if part of the total health expenditure is always funded by private insurance and out-of-pocket payments, almost the entire amount of inpatient health expenditure is financed publicly. The total expenditure on inpatient care (PPP$ per capita) in the EU follows, on average, a growing positive trend. The exception is Greece, where data available show that this indicator varies negatively (–19%).
Pharmaceutical expenditure includes the consumption of prescribed medicines, over-the-counter and other medical non-durable goods. One of the indicators taken into consideration for 2016 was the expenditure on pharmaceuticals and other medical non-durable goods, as percentage of current health expenditure. The countries that registered the lowest rates of this indicator are Denmark (7%), The Netherlands (8%), Luxembourg (9%) and Sweden (10%) whereas the highest ones were Greece (26%), Lithuania (27%), Latvia (28%) and Hungary (29%).
Between 2006 and 2016, the percentage of pharmaceutical expenditure on total current health expenditure has generally declined in all of Europe. In 2016, the total pharmaceutical expenditure was encompassed between 335 PPP$ and 369 PPP$ per capita in Denmark and Poland respectively, and 777 PPP$ and 1080 PPP$ per capita in Germany and Switzerland. At least half of it was held by the public sector in all countries except Denmark (44%), Latvia (35%) and Poland (34%) and Lithuania (33%). The highest values in 2016 were in Germany (84%), Luxembourg (80%), Ireland (77%), France (76%) and Slovakia (71%). In 2016 the pharmaceutical expenditure in PPP$ per capita held by the public sector was encompassed between 124 in Poland and 655 in Germany.
Chart 5 explores the relationship between the trend of the total and the public pharmaceutical expenditure between 2006 and 2016. In a group of outlier countries (upper right part of the chart) encompassing Estonia, Latvia and Lithuania, both the public and the total spending varies substantially. In Portugal, Luxembourg and Greece, the same indicators varied negatively.
From 2006 and 2016 in the EU, the total pharmaceutical expenditure decreased more than the public pharmaceutical expenditure, which decreased as well but at a slower pace. This suggests that a progressively larger part of the total pharmaceutical expenditure pertains to the private sector. This shift may also indicate that the ‘willingness to pay’ and the consumption of pharmaceuticals by private owners are increasing.
In the last 15 years, healthcare reforms or other initiatives implemented all over Europe aimed at rationalising the use and provision of hospital care, improving its quality and appropriateness, and reducing its costs. The number of hospital facilities decreased in most of the countries while the number of hospital beds dropped off on average. These reforms/initiatives also resulted in a broad reduction of acute care admissions and length of stay, as well as in improvements in the occupancy rate of acute care beds.
During these years, almost all European countries had changes in their hospital provision patterns, and major efforts were made to deliver better services, increase quality, and improve efficiency and productivity. The streamlining of care delivery started from a sharp reduction in the size of secondary care institutions and moved towards more integrated and efficient patterns of care, which might result in completely overcoming the hospital-centric model of care in the future.
This was possible thanks to a package of financial and organisational measures addressed to improve coordination and integration between the different levels of care, increase the use of day-hospital and day-surgery and introduce new and more efficient methodologies of hospital financing in order to incentivise appropriateness (for example, the replacement of daily payments – known to encourage longer hospitalisation – by prospective payment).
In most European countries, these policies led to changes in the management of patients within hospitals and offered a possibility to reduce the number of acute care hospital beds. Only the bed-occupancy rates, registered more disparate trends across Europe, depending also from the demographic and epidemiological structure of population and from the specific organisation of local, social and healthcare systems, i.e. the structure of primary care, the presence and the efficiency of a gate-keeping system, the modality of access to secondary care, availability of home care and development of community care.
In 2016 there were on average 2.7 hospitals for 100,000 inhabitants, ranging from 1.4 in Slovenia to 4.8 in Finland. Moreover, there were on average 484 hospital beds for 100,000 inhabitants, ranging from 234 in Sweden to 806 in Germany.
Between 2006 and 2016 little change in the number of hospitals was registered in Luxembourg (–2), Slovenia (0) and Czech Republic (+3) (Chart 6). Major increases were registered in the United Kingdom (+175), France (+182), Poland (+229) and The Netherlands (+350). Major decreases were registered in Germany (–259), Italy (–193) and Ireland (–92).
In the same period, the total number of hospital beds decreased by 14% ranging from –41% in Finland (which means 302 beds cut every 100,000 inhabitants) and –3% in Germany (which means 24 beds cut every 100,000 inhabitants) (Chart 7). Positive variations have been registered in Poland (+2%), Austria (+2%) and Luxembourg (+4%). In Poland, the total number of hospital beds per 100,000 increased of 17 units. In Luxembourg and Austria, such increase corresponded to a reduction of the total number of beds per 100,000 inhabitants of 87 and 24 units, respectively.
In several countries, the decrease in the total number of beds was accompanied by a slight increase in the number of private inpatient beds, which are inpatient beds owned by not-for-profit or for-profit private institutions. In 2016, in most of the countries where the data are available, the beds in private owned hospitals as a percentage of all beds ranged from 1% in Slovenia and Lithuania to 38% in France. The figures were higher in Cyprus (46%), Germany (59%) and The Netherlands (100%).
The rate of acute care hospital beds for 100,000 inhabitants in 2016 in the EU ranged from 215 in Sweden to 606 in Germany. The highest figures were seen in Belgium (503), Austria (555) and Lithuania (581), whereas the lowest figures were in Spain (241), Denmark (252) and Italy (262).
Between 2006 and 2016, the number of acute care hospital beds per 100,000 population reduced on average by 13% in EU. The most significant decreases were in Latvia (–36%), Denmark (–32%) and Hungary (–28%). The only exceptions were Ireland (+1%), The Netherlands (+1%) and Poland (+6%).
The reduction in the number of hospital beds relates especially to the public providers. In the countries where data are available, this trend is associated with an increase of hospital beds in private organisations. This is the case in Austria, Bulgaria, Croatia, Czech Republic, Germany, Latvia, Lithuania, Poland, Portugal and Romania. The countries that registered a decrease in both categories are Cyprus, Denmark, Estonia, Finland, France, Greece, Hungary, Italy, Malta, Slovenia and Spain.
The number of acute care discharges involves the entire pathway of hospitalisation of a patient, who normally stays in hospital for at least one night and then is discharged, returns home, is transferred to another facility, or dies. Curative care comprises health care contacts during which the principal intent is to relieve symptoms of illness or injury, reduce the severity of an illness or injury, or to protect against exacerbation and/or complication of an illness or injury that could threaten life or normal function. Curative care includes all components of curative care of illness (including both physical and mental/psychiatric illnesses) or treatment of injury; diagnostic, therapeutic and surgical procedures and obstetric services. It excludes rehabilitative care, long-term care and palliative care.
In 2016, the rates of acute care hospital discharges in the European countries were quite dissimilar, ranging from 10% in the Netherlands and Italy to 24% in Austria and Denmark.
The average length of stay measures the total number of occupied hospital bed-days, divided by the total number of discharges. In 2016, the average length of stay in acute care hospitals ranged from 5 bed-days in the Netherlands and Greece to 8 bed-days in Germany and Luxembourg.
Between 2006 and 2016 the number of inpatient discharges in acute care hospitals remained on average stable; however, the indicator varied consistently across the EU member states. Major decreases were registered in Latvia (–36%), Hungary (–25%) and Italy (–23%), whereas major increases were registered in Germany (+17%), Poland (+20%) and Switzerland (+25%).
The link between the rate of admissions and the length of stay can be a very sensitive issue for hospitals, because it is commonly acknowledged that too short a length of stay may increase the risk of re-admissions, resulting in consequent waste of resources not only for the hospital but also for the patients and their carers. At the same time, staying too long in a hospital may indicate inappropriate settlements of patients, causing also a waste of resources.
Chart 11 compares the rate of hospital discharges and the average length of stay in acute care hospitals in 2016. The last updated data shows that the average European figures corresponds to a mean rate of discharges by 16% and a mean length of stay of 6 days for acute care hospitals. Chart 11 shows that both indicators are higher than the EU average in Austria, Belgium, Finland, Slovenia, Poland, Slovakia, Lithuania and Germany.
The bed occupancy rate represents the average number of days when hospital beds are occupied during the whole year and generally mirrors how intensively hospital capacity is used.
Between 2006 and 2016, the average rate of acute bed occupancy decreased in Europe. The reduction was encompassed between –8.4 p.p. and –8.2 p.p. in the Czech Republic and The Netherlands, respectively, and –0.5 in Estonia and Italy. The increase ranged between +0.8 p.p. and +2.8 p.p. in the United Kingdom and Germany, respectively. In Ireland, the increase was 7.5 p.p. These large variations are usually due to changes in the number of admissions, average length of stay and the extent to which alternatives to full hospitalisation have been developed in each country (Chart 12).
Despite the growing interest in self-treatment and the growing role of eHealth and mHealth, health workers remain the crucial component of health systems, providing health services to the population. Jobs in the health and social sector now account for more than 10% of total employment in many OECD countries. Despite the tendency for the number of health care workers to grow in the last 15 years, policy makers are raising issues about the upcoming retirement of the ‘baby-boom’ generation of doctors and nurses, exacerbating the workforce shortage in the health field. Health workforce concerns have shifted from worries on shortages towards issues related to the right skill-mix, to better respond to evolving population health needs (Health Workforce Policies in OECD Countries, OECD March 2016).
According to the European Commission supplement to the quarterly review on ‘Health and social services from an employment and economic perspective’ (December 2014), there are large imbalances in skills levels, and working patterns and recruitment and retention are conditioned by demanding working conditions. The financial constraints are leading in most European countries to a decrease in the resources available for healthcare professionals, reducing the possibilities of hiring new staff. Additionally, several countries, especially in Central and Eastern Europe, are experiencing migrations of their healthcare workforce.
European countries, European Organisations and EU institutions are discussing possible impacts and achievable solutions to these issues. Interestingly, several countries are shifting competences from doctors to nurses, creating new educational pathways and bachelor degrees addressed to nurses. In many cases nurses and general practitioners acquire new skills and competences relieving the burden of hospital care by enforcing primary care institutions and community services.
The trends described above, are likely to have major impacts on the hospital sector, since inpatient care alone absorbs about a third of the healthcare resources and since the hospital sector gives work to more than half of active physicians.
In 2016, the share of practising nurses per 100,000 registered the lowest values in Greece (325), Latvia (464), Poland (516), Spain (551) and Italy (557) (Chart 15). The highest values belong to Germany (1285), Finland (1426), Denmark (1690) and Switzerland (1702). In the same year, the lowest share of practising physicians was registered in Poland (242), the United Kingdom (278), Luxembourg (288), Ireland (294) and Slovenia (301), whereas the highest values were seen in Germany (419), Switzerland (425), Sweden (427), Lithuania (447) and Austria (513) (Chart 14). Between 2006 and 2016, the number of both practising nurses and physicians increased by 15% in EU, according to information available.
These figures provide evidence of the policies implemented, or at least the trends for the management of healthcare professionals, especially concerning the allocation of resources and responsibilities between physicians and nurses. In the EU, the average rate of nurses per physicians is about 2.4 points. In 2016, the highest values are in Denmark (4.6), Finland (4.4), Luxembourg (4.1), Switzerland (4.0) and Belgium (3.6). In these countries, there is a high shift of competencies from physicians to nurses. Conversely, in countries where the values are lowest – such as Lithuania (1.7), Austria (1.6), Latvia (1.4), Spain (1.4) and Italy (1.4) – physicians continue to perform most of the clinical activities.
In 2016, according to last data available, physicians per 100,000 inhabitants working in hospitals (full or part time) were approximately 50%–60% of the total, with the highest rates registered in Lithuania (66%), Estonia (68%), Switzerland (74%) and France (83%). By contrast, the lowest values were seen in Belgium (24%), the Netherlands (39%), Poland (45%) and Finland (46%).
The most relevant positive variations on the number of physicians working in hospital between 2006 and 2016 were registered in Switzerland (+49%), Germany (+34%) and Hungary (+32%). By contrast, this indicator registered negative variations in Poland (–2%) and Greece (–9%).
In 2016, the average number of physicians and nurses graduated for every 100,000 inhabitants were respectively about 14 and 42 in the EU; however, the values across countries were quite different. The number of medical graduates per 100,000 inhabitants ranged from 9 in France and Greece to 24 and 22 in Ireland and Denmark, respectively (Chart 16). The number of nurses graduated per 100,000 inhabitants ranged from 15 and 16 in Luxembourg and the Czech Republic to 99 and 104 in Switzerland and Denmark (Chart 17).
Compared with 2006, the number of medical graduates per 100,000 inhabitants in the EU registered an overall positive variation. The countries that registered the highest increases were Portugal (+109%), Belgium (+137%), Slovenia (+156%) and Latvia (+158%). Minor positive variations occurred in Germany (+11%), Sweden (+11%), Denmark (+12%) and Estonia (+13%). Decreases happened in Greece (–37%) and Austria (–16%). The number of nurses graduated per 100,000 inhabitants registered different trends across the EU. Major positive variations were registered in Belgium (+75%) and Switzerland (+63%), whereas minor positive variations were registered in Latvia (+3%) and Hungary (+5%). Negative variations ranged from –1% and –4% respectively in Poland and Austria, to –24% and –25% in Slovakia and Portugal. The most relevant decrease was registered in the Czech Republic (–68%) in the same years.
Dr Joaquín Casariego discusses Janssen’s solid tumour portfolio and pipeline, highlighting their commitment to improving outcomes in the solid tumour space.
Having worked in the pharmaceutical industry for over 20 years, I joined Janssen in 2016 as EMEA Medical Director Oncology and began my current role as EMEA Therapeutic Area Lead Oncology in 2019.
At Janssen we have been active in oncology for over 30 years, significantly contributing to improved patient outcomes in the solid tumour space. Our driving passion is to bring innovative medicines to patients at the earliest stages of their disease where there is maximum potential for cure and, when not possible, have the highest impact on patient outcomes.
Within the last seven years, we have recognised numerous milestones across our solid tumour portfolio in Europe – from launching two treatments in prostate cancer, abiraterone acetate (Zytiga®)1 and apalutamide (Erleada®),2 to seeing abiraterone acetate included in the World Health Organization (WHO) Essential Medicines List for the treatment of metastatic castration-resistant prostate cancer (mCRPC).3
In addition to our approved products in prostate cancer, we have a strong late-stage pipeline in solid tumours. Our focus is on discovering best-in-class, innovative medicines for cancers where patient and their families’ needs are greatest. We do this by pursuing the cancer types and sub-types that we know best, and where we can achieve the highest impact – both in transforming patients’ lives today and in moving closer to a cure tomorrow.
This is demonstrated by the ongoing Phase II GALAHAD study, exploring niraparib, an orally-administered poly-ADP-ribose-polymerase (PARP) inhibitor for third-line treatment of adult men with mCRPC with bi-allelic DNA-repair defects (DRD).7 There is currently a high unmet need in this particular patient group and we were delighted to share positive preliminary results from the GALAHAD study earlier this year.7 In addition, the Phase III MAGNITUDE trial is exploring the efficacy and safety of the novel combination of niraparib plus abiraterone acetate as first line treatment of mCRPC patients with and without DRD.10
In recent years, drug development in early disease stages of cancer has been limited by a lack of clinically relevant endpoints. As our research explores earlier stages there is a greater need to find endpoints to replace long term endpoints such as overall survival. To address this, Janssen created a pioneering development strategy to address how our medicines are evaluated and approved. Although overall survival is still considered the gold standard of interventions in trials, together with clinical experts in the field, we are developing clinical trial programmes that maximise the impact of novel surrogate and intermediate endpoints with a view to redefine patient outcomes at all disease stages.11

Our use of novel efficacy endpoints has supported several submissions to regulatory bodies including the submission and approval of apalutamide for patients with high-risk non-mCRPC (nmCRPC).1 This approval was based on the Phase III SPARTAN study, which showed statistically significant improvement versus placebo for the primary endpoint of metastasis-free survival.12

Not only is it important to develop innovative medicines for the treatment of cancer, but it is vital that patients have the opportunity to get the treatment most likely to benefit them. As such, a growing number of our pipeline products are centred in a precision medicine approach, are biomarker-based and are identified by a companion diagnostic. As part of our commitment to bring the right treatment, to the right patient, at the right time, for the right outcome, we are developing a biomarker test to identify patients with urothelial cancer and FGFR alterations. This will identify those most likely to benefit from targeted therapy with erdafitinib, a first and best in class FGFR inhibitor which is available in the US.15,16 We believe that advanced diagnostics can deliver actionable information and cost-effective solutions to guide targeted, individualised use of our therapies in the clinic.
A similar approach is being followed for the treatment of lung cancer with EGFR alterations, through assets currently in the pipeline. At Janssen we are partnering with numerous experts to bring to life our commitment to precision medicines. These partnerships are exploring computational tools, artificial intelligence and big data that will result in state-of-the-art, real-world evidence to enable superior decision-making for diagnostics and disease management.
Using these approaches, we need to characterise the mechanisms of the tumour cells that lead to drug resistance and develop tests to detect abnormalities in the androgen receptor. These methods will enable us to diagnose and eventually stop cancer earlier in the disease – this process is known as cancer interception. Janssen researchers are currently exploring cancer interception in both multiple myeloma and prostate cancer.
It certainly is a thrilling time in oncology and the progress is being driven by passionate individuals that I am delighted to call my peers and colleagues. I am personally hugely excited to be playing a part in the future of cancer medicines.
More information about the Janssen Oncology Solid Tumour portfolio can be found on the website www.janssen.com/emea.
References
1 European Medicines Agency. ZYTIGA Summary of Product Characteristics. www.ema.europa.eu/en/documents/product-information/zytiga-epar-product-i… (accessed August 2019).
2 European Medicines Agency. ERLEADA Summary of Product Characteristics. www.ema.europa.eu/en/documents/product-information/erleada-epar-product-… (accessed August 2019).
3 World Health Organization. WHO Model List of Essential Medicines. https://apps.who.int/iris/bitstream/handle/10665/325771/WHO-MVP-EMP-IAU-… (accessed August 2019).
4 European Medicines Agency. Zytiga. www.ema.europa.eu/en/documents/overview/zytiga-epar-summary-public_en.pdf (accessed August 2019).
5 Janssen. 2017. European Commission extends license for Janssen’s ZYTIGA® plus prednisone/prednisolone to include earlier stage prostate cancer patients. www.businesswire.com/news/home/20171120005575/en/European-Commission-Ext… (accessed August 2019).
6 Janssen. 2019. Janssen announces European Commission approval of ERLEADA® (apalutamide) for non-metastatic castration-resistant prostate cancer patients who are at high risk of developing metastatic disease. www.businesswire.com/news/home/20190116005454/en/Janssen-announces-Europ… (accessed August 2019).
7 Smith M et al. Phase 2 study of niraparib in patients with metastatic castration-resistant prostate cancer (mCRPC) and biallelic DNA-repair gene defects (DRD): preliminary results of GALAHAD. Abstract #202. Presented at ASCO GU 2019.
8 Chi K et al. First results from TITAN: A Phase III double-blind, randomized study of apalutamide (APA) versus placebo (PBO) in patients (pts) with metastatic castration-sensitive prostate cancer (mCSPC) receiving androgen deprivation therapy (ADT). J Clin Oncol 2019;(suppl 37):abstr 5006.
9 Janssen. 2019. Janssen seeks to expand use of ERLEADA® (apalutamide) in the treatment of patients with metastatic hormone-sensitive prostate cancer. www.businesswire.com/news/home/20190604005600/en/Janssen-Seeks-Expand-ER… (accessed August 2019).
10 ClinicalTrials.gov. Identifier: NCT03748641. A Phase 3 Randomized, Placebo-controlled, Double-blind Study of Niraparib in Combination With Abiraterone Acetate and Prednisone Versus Abiraterone Acetate and Prednisone in Subjects With Metastatic Prostate Cancer.
11 Bennett C. A Powerful Force: Novel endpoints speed up drug development. OBR Oncology 2018;11:4. http://obroncology.com/article/a-powerful-force-novel-endpoints-speed-up… (accessed August 2019).
12 Smith RM et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med 2018;378:1408–18.
13 Sandler, et al. ATLAS: A randomized, double-blind, placebo-controlled, phase 3 trial of apalutamide (ARN-509) in patients with high-risk localized or locally advanced prostate cancer receiving primary radiation therapy. J Clin Oncol 201;7(suppl 15):abstr TPS5087.
14 Taplin M-E et al. PROTEUS: A randomized, double-blind, placebo (PBO)-controlled, phase 3 trial of apalutamide (APA) plus androgen deprivation therapy (ADT) versus PBS plus ADT prior to radical prostatectomy (RP) in patients with localized high-risk or locally advanced prostate cancer. J Clin Oncol 2019;suppl 37:abstr TPS5100.
15 Erdafitinib [package insert]. Janssen Pharmaceuticals, Inc; 2019 (accessed August 2019).
16 Loriot Y et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 2019;381:338–48.
Date of prep August 2019
Job code CP-105578

Mr Nikolaus Koller
HOPE Governor

Mrs Ulrike Schermann-Richter
HOPE Liaison Officer, Federal Ministry of Labour, Social Affairs, Health and Consumer Protection
Could you describe the last hospital and/or healthcare reforms implemented in your country in the past 5 years?
Due to the federalist structure of the state and the self-governed system of compulsory insurance, the Austrian healthcare system is characterised by a fragmentation of responsibilities for regulating, planning, financing and providing healthcare between the different legislative as well as administrative levels (federal and regional governments) and the social insurance funds.
The rather complex blend of different decision-making structures favours frictional losses and inefficiency. However, streamlining the historically grown constitutional competences was never feasible.
The growing pressure on health systems during, and in the aftermath of the economic crisis, gave impetus for a reform that aimed to overcome the traditional fragmentation and to improve coordination and policy coherence. In 2013, thus, a target-based health governance system for the joint management of the structure, organisation and financing of the Austrian healthcare system was established. The system is based on domestic state treaties, which are concluded for a limited period of time (4–5 years) between the federal and the regional governments with the involvement of the social insurance institutions, while leaving the constitutional division of powers and responsibilities unchanged.
In 2017, the second Federal Target-Based Governance Agreement was signed. It sets four strategic goals that are further specified in eleven objectives, concrete indicators and targets and almost 80 measures to be taken at federal and/or regional level over a period of five years. The strategic goals are:
In December 2018, a reduction of the number of social insurances funds was decided by the Parliament inter alia by merging the nine regional health insurance funds into a single one covering almost the 80 % of the population. Savings in administration and efficiency gains are expected.
Could you present two/three elements on the impact of such reforms on hospital and/or healthcare sectors that your organisation/country has identified?
A significant reform activity was the development of a new approach to the provision of primary healthcare, which is traditionally provided by individual general practitioners working alone in private practices, contracted to one or more social insurance funds. In 2017, the Primary Health Act was adopted, laying down uniform requirements for the new primary care across Austria. In the same year, the establishment of a total of 75 multi-professional and interdisciplinary primary care centres and networks, respectively, by the end of 2021 was agreed. The new primary care units are to be achieved by attracting existing GPs and other health professionals working together in teams, thus being able to provide more comprehensive services and more opening hours for the patients while, coincidently, the working time is better distributed between the team members, favouring a better work–life balance.
A few primary care units are already in operation and are well accepted by the patients. The medical staff also seems to be satisfied. In order to promote the new concept, appropriate contractual agreements are under negotiation and start-up programmes are being developed. Strengthening primary care is crucial as the Austrian health system has still a strong focus on hospital (inpatient) care.
The introduction of the Electronic Health Record (ELGA), together with its e-medication and e-report applications, is making good progress. Since the end of 2015, ELGA is being rolled-out in stages, starting with public hospitals. Between March 2018 and September 2019, ELGA and e-Medication are being made available step by step to doctors in private practices, group practices, pharmacies and outpatient clinics. The aim of ELGA is to reduce organisational barriers, improve coordination and strengthen patients’ rights.
Whether the recent reform of the organisational structure of the health insurance funds will affect the healthcare system will only become apparent in the coming years.
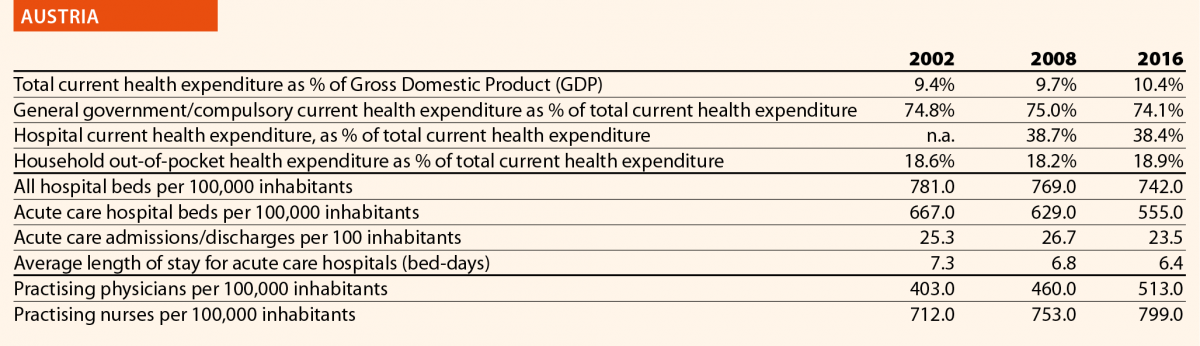

Mrs Eva M Weinreich-Jensen
HOPE President, Danish Regions
Could you describe the last hospital and/or healthcare reforms implemented in your country in the past 5 years?
A major reform of the Danish healthcare sector took place in 2007. Following the reform, the number of hospitals was heavily reduced and concentrated, and the specialised hospital services were centralised. Whatever has been done after 2007 has been an attempt to add to, and to adjust, the 2007 reform.
One example is the National Strategy for Personalised Medicine 2017–2020, involving a national infrastructure working on both treatment and research. Moreover, regional data support centres will be established, providing researchers, clinicians and health personnel with reliable data.1
Another example is the National Digital Health Strategy 2018–2022, based on 25 specific initiatives empowering patients by giving them a complete overview of their digital health data and by offering them a digital copy of their health records.2
A third example is the National Strategy for Cyber and Information Safety in the Healthcare Sector 2019–2022, which aims to make the digital system in health secure and trustworthy, given the amount of patient data handled.
At the moment, the Danish Region is looking at a national wish to reform the outpatient system and to make patients’ paths easier and more flexible while ensuring a better coordination of care. The focus shall be also on how to face the demographic challenges by changing the structural mind-set. This may come in the form of defining health communities around 21 acute hospitals; an increased cooperation between general practitioners and the hospitals and an overall strengthening of the pre-hospital effort and the acute functions. The overall trend is a change from focus on treatment activity and volume, to prevention and quality treatment (outcomes). The basic goal is to give patients a better service by increasing the possibility to diagnose and treat them at home or in primary settings, while, at the same time, reducing the burden to the hospitals.
Could you present two/three elements on the impact of such reforms on hospital and/or healthcare sectors that your organisation/country has identified?
Adjusting the incentives of the system is an ongoing process, especially when it comes of adapting to a reform. The focus is now on changing a silo-based approach to treatment, adopting a more holistic view empowering patients; improving patient pathways; creating a strategy for digital development; investing in personalised medicine; providing transparency to patients for what concerns their health data; and encouraging initiatives on cybersecurity and the use of new technology. Health workforce needs to be supported and trained on how to face these changes and challenges.
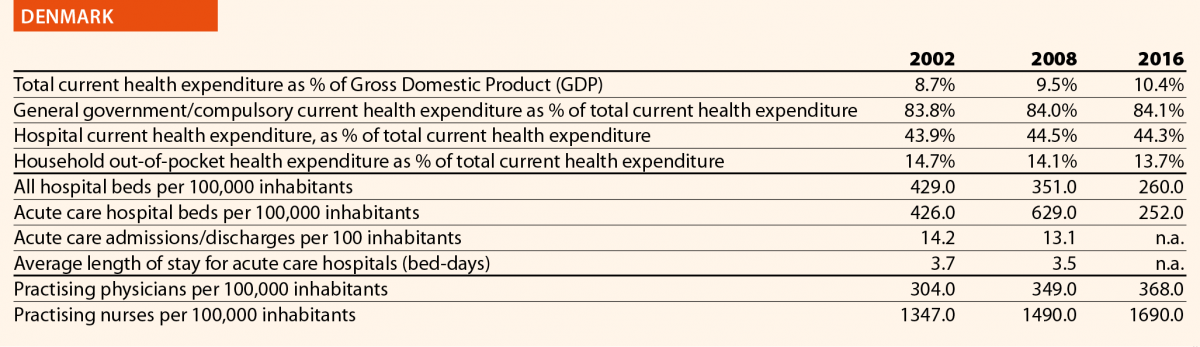

Mr Urmas Sule
HOPE Vice-President, Estonian Hospitals Association
Could you describe the last hospital and/or healthcare reforms implemented in your country in the past 5 years?
In 2017, the Estonian government made a historic and long-awaited decision about healthcare system financing. The Estonian healthcare system is mainly publicly funded through solidarity-based mandatory health insurance contributions in the form of an earmarked social payroll tax (13% of wages). The health insurance system covers about 94% of the population.
Contributions are related to employment, but the share of non-contributing individuals covered by the Health Insurance Fund (for example, children and pensioners) represents more than half of the insured. Health Insurance Fund revenue base has been dependant on the amount of working people paying social tax, but not unlike other countries, Estonia is also facing the challenges of an ageing population.
So finally, in 2017, in close cooperation with the healthcare social partners, the Estonian government promoted the initiative of expanding the revenue base of the health system, which has been a longstanding challenge. The government decided to broaden the Health Insurance Funds revenue base by gradually increasing a state contribution into the Health Insurance Fund on behalf of pensioners, starting from 2018. By 2022, this contribution should reach 13% of average pensions, which is the same rate as the current earmarked social health insurance contribution from payroll tax. This will not solve all problems, but it is definitely a step in the right direction. Thanks to this decision it was possible to renew the nationwide collective agreement in the health sector.
This decision impacts also on other services previously covered by the state budget. For example, emergency care (including for non-insured people), ambulance care and IVF are, or will, be under the responsibility of the Health Insurance Fund. The aim of the change was to make the healthcare system more efficient by strengthening the purchasing role of the Health Insurance Fund and making it responsible for financing health services for the whole population and not only for the insured.
Estonia is continuously focusing on developing e-health solutions to improve the quality and availability of healthcare services. In 2019, Estonia will launch a statewide digital project to increase health service provision transparency and availability.
Could you present two/three elements on the impact of such reforms on hospital and/or healthcare sectors that your organisation/country has identified?
The previously mentioned reform will not lead to a significant increase in total public health expenditure, but it will consolidate previously fragmented service funding under the Health Insurance Fund. Consequently, the Health Insurance Fund budget will increase significantly in the following years, but at the same time it will also have more responsibilities. However, the funding of the health system will be more logic. In summary, this reform is a good example of Estonia adapting to new situations and challenges, finding innovative solutions, but there is still work to be done in achieving a more sustainable financing system of healthcare.
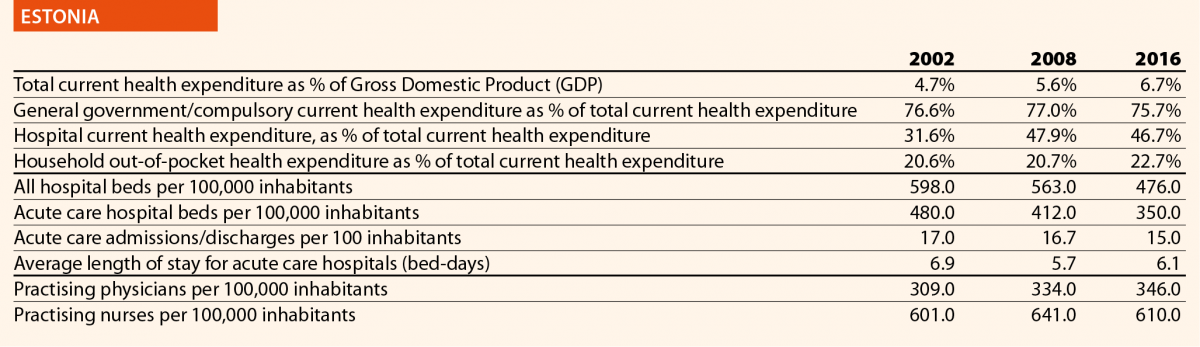

Mrs Hannele Häkkinen
HOPE Governor, The Association of Finnish Local and Regional Authorities
Could you describe the last hospital and/or healthcare reforms implemented in your country in the past 5 years?
One of the initiatives implemented in recent years was the division of healthcare services provided between secondary hospitals and centralised hospitals. In particular, centralised hospitals are now in charge of demanding and providing rare treatments and diagnostics, for example, surgical operations including hip and knee replacement, as well as childbirths. The five university hospitals offer the most demanding treatment. The goal is to guarantee equal access to services, sufficient skills and knowledge to health professionals, patient safety as well as to curb the cost growth.
A second initiative is the introduction of joint emergency care units (primary and secondary care) in central hospitals and the division of work between nurses and doctors in primary care and emergency care in hospitals.
Finally, the Health and Social Services Reform is ongoing. The goal is to put primary care, secondary care and social care under one umbrella. At the moment the municipalities are responsible for organising health and social services. This responsibility will be transferred to 18 new counties on 1 January 2021. The law proposal is in Parliament but it is uncertain as to whether it will be approved because there will be parliamentary elections in April 2019.3
Could you present two/three elements on the impact of such reforms on hospital and/or healthcare sectors that your organisation/country has identified?
The goals of the centralisation of the most demanding and rare treatment to fewer hospitals are better cost-efficiency, better quality of care and treatment and better patient safety.
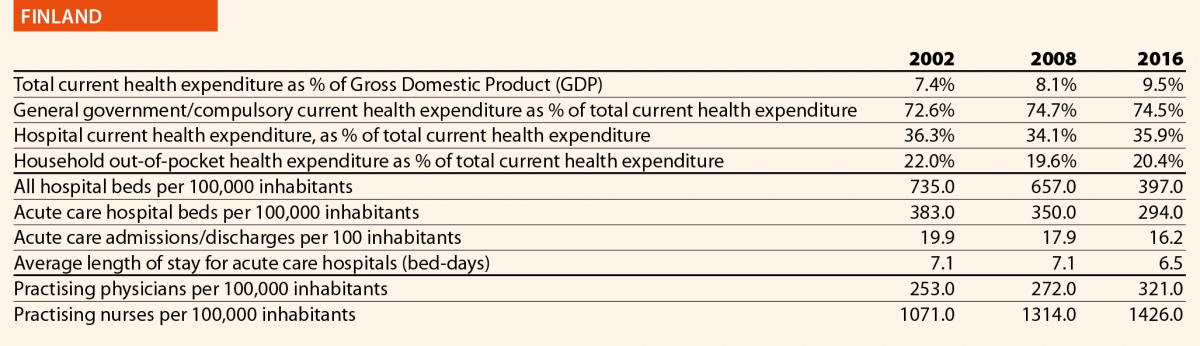

Mr George Baum
HOPE Governor, German Hospital Federation
Could you describe the last hospital and/or healthcare reforms implemented in your country in the past 5 years?
In the last 5 years, political priorities have focussed on the continuous development of quality assurance and of the reimbursement systems for healthcare providers. Additionally, a great importance has been given to measures to face the challenge of health workforce shortage.
In 2015, the so-called Hospital Structures Act (Krankenhausstruktur-Gesetz) enforced quality assurance in hospitals; improved financing of nurses and strengthened patient safety as regard nosocomial infections by fostering a hygiene support programme. This act entered into force on 1 January 2016 and included the following key aspects:
The training of nurses has been subject of reform with the 2015 Nursing Professions Reform Act (Pflegeberufereformgesetz). Training for nurses, paediatric nurses and long-term care nurses have been merged into a unique programme.
With a law on the continuous development of provision and reimbursement of psychiatric and psychosomatic care in the year 2016, a services-oriented financing for hospitals has been introduced. Transparency has been enhanced and a better coordination of inpatient and ambulant services enabled.
In May 2018 the most important hospital act in the running legislative period for hospitals has been forecasted. With the Care Personnel Strengthening Act (Pflegepersonalstärkungsgesetz), the general status of nursing and long-term care shall be improved. It is focussed on the full reimbursement of labour costs for care personnel and its extraction of the DRG-system starting with the year 2020. Additionally, partners of the self-governance system (health insurances and services providers) have been mandated to extend the already existing minimum levels for care personnel to further departments in the hospitals. This law came into force at 1 January 2019.
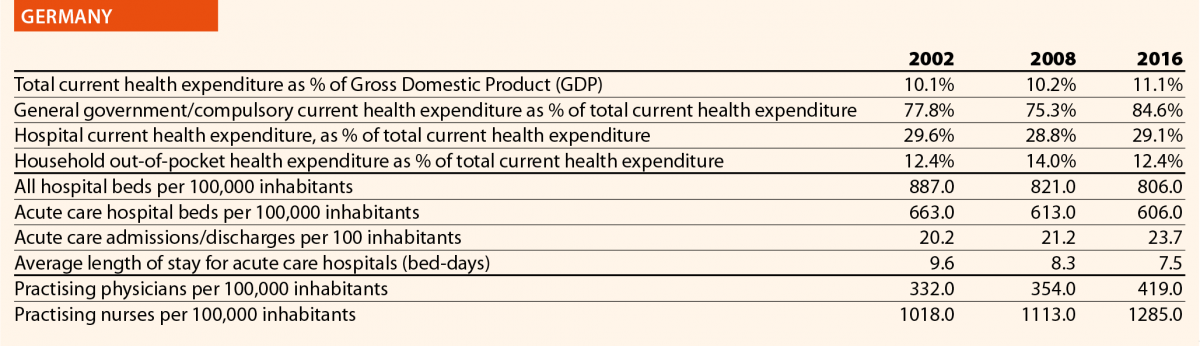

Mr Domenico Mantoan
HOPE Governor, Veneto Region
Could you describe the last hospital and/or healthcare reforms implemented in your country in the past 5 years?
In the past 5 years, as regards the hospitals, the most important law is the National Decree n.70/2015. It states the new hospitals standards (structural, technological, organisational and clinical) that have to be applied in each Italian Region. The decree states that each Italian Region has to create a network of acute hospitals, based on three hierarchical levels: basic hospitals (serving a population of 80,000 – 150,000 inhabitants); first level hospitals (serving a population of 150,000–300,000 inhabitants); second level hospitals (serving a population of 600,000–1,200,000 inhabitants). It states, also, the criteria for the number and type of clinical departments. For example, cardio-surgery departments have to serve a population between 600,000 and 1,200,000 inhabitants. The decree states also the operative standards; for example, each breast surgery department has to treat at least 150 cancer cases per year.
As regards general aspects of healthcare, a National law was approved in January 2017, regarding the new so-called essential levels of assistance (LEA – Livelli Essenziali di Assistenza). The LEA represents the minimum levels of health services that have to be provided by the Italian National Health System (NHS) through public national funding. Some LEA must be provided for free (that is, acute hospital inpatient services) whereas others need to be covered through a co-payment from the patient. New LEA legislations have come after a long period without any national update. In fact, the last (and first) national LEA legislation was in 2001.
As regards the healthcare reform, an innovative regional reform commenced in Veneto in 2016. It is based on two main topics: the reduction of the number of Health and Social Local Trusts (HSLTs) and the creation of an innovative Trust, called Zero Trust (Azienda Zero), which absorbed some functions – mainly administrative – that were previously performed by other Trusts or regional offices. The Zero Trust does not provide any clinical services. It was conceived with the aim of increasing efficiency and productivity through a more effective use of resources, following a unified approach.
Could you present two/three elements on the impact of such reforms on hospital and/or healthcare sectors that your organisation/country has identified?
Regarding national hospitals standards, each region had to reorganise its inpatient settings by reducing the number of acute hospital beds. The organisational impact was important because outpatient care and home care were strengthened. Country hospitals were implemented too. Hospitals teams are encouraged to work in network. Clinical networks (oncological, cardiologic and emergency networks) were created. Some departments were closed such as the surgery departments being under standard in terms of number of interventions.
As regards new LEA legislation, it has introduced some free vaccinations that previously were not covered, namely vaccinations against papilloma virus, meningococcus and pneumococcus. The introduction of new screening tests in newborns, such as tests for congenital deafness and cataract, had an important impact. For disabled individuals, new information technology was introduced. For example, eye gaze control systems and speech generating devices are covered for severe stages of paralysis (such as in severe amyotrophic lateral sclerosis). At the same time, the new LEA restricted the coverage of some types of treatment, particularly dental care.
Veneto Region went through a reform reducing the number of Trusts from 21 to nine. The impact of such reform was important because this resulted in a new distribution of competences. The Zero Trust centralised all the administrative functions such as nursing recruitment, large procurement, regional education and health technology assessment. The centralisation of ICT services offers a new important challenge for storing and managing health and organisational information in a unique digital archive.
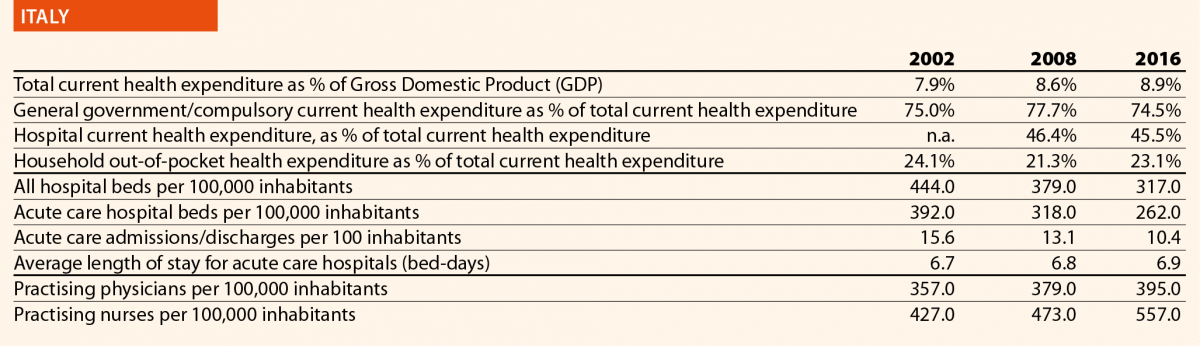

Mr Carlos Pereira Alves
HOPE Governor, Portuguese Association for Hospital Development
Could you describe the last hospital and/or healthcare reforms implemented in your country in the past 5 years?
Primary healthcare reform4
In recent years, the Primary Healthcare Network has been strengthened and expanded, through the deepening of the Primary Health Unit’s model and performance, with the objective of increasing the NHS response and assigning a family health team to all residents.
Integrated responsibility centres
In 2018, the model of Integrated Responsibility Centres (CRIs) that are organic intermediate management structures, dependent on the NHS public entities boards of directors was defined.5 The CRIs have functional autonomy and establish an economic and financial assistance commitment, following the presentation of an action plan that defines the healthcare programme for a period of three years. The CRIs are created to ensure the development of best clinical practices focused on the users’ needs; foster clinical governance processes and therefore care quality improvement; increase the accessibility and timely response of the NHS to citizens; mobilise the installed capacity in the NHS public network; promote the autonomy and involvement of professionals holding managerial responsibilities; and increase NHS professionals’ productivity and satisfaction levels, linking the institutional and financial incentives allocation to the performance effectively achieved.6
Affordable and quality palliative care7
The Strategy for the Development of Palliative Care resulted in a National Network of Palliative Care, integrated in the NHS and implemented at all levels of healthcare.
Digital transformation (E-Health)
In 2017, the National Strategy for Health Information Ecosystem 2020 was approved,8 which aims to increase citizens’ interaction with health technology. To this end, it provides the availability of multiple digital service platforms that allow information access and sharing, but also the simplification and informatisation of registries and processes in the NHS till 2020 such as E-Prescription, Electronic health record, Portal SNS9 and mobile applications (MySNS,10 MySNS TEMPOS,11 MySNS CARTEIRA12).
Clinical Academic Centres and University Hospitals
In 2018, Clinical Academic Centres were created and pilot projects of University Hospitals were established.13 Clinical academic centres are consortiums and associations of healthcare units (for example, hospitals), higher education institutions (for example, universities) and/or research institutions (public or private). These centers are dedicated to the promotion of integrated healthcare, teaching, clinical research and translation. With the pilot projects of university hospitals, some established university hospital centres were reorganised to improve their working processes and functions.
Could you present two/three elements on the impact of such reforms on hospital and/or healthcare sectors that your organisation/country has identified?
The reinforcement and expansion of the Primary Healthcare Network significantly improved primary healthcare, in terms of accessibility and quality. On one hand, this is visible by the increase in the number of Portuguese with family doctor (93 % in 2017); and on the other hand, by the number of medical appointments, which is over 30 million per year. In January 2018, the highest number of physicians, nurses and diagnostic and therapeutic technicians, working in the NHS, in the last ten years was registered.
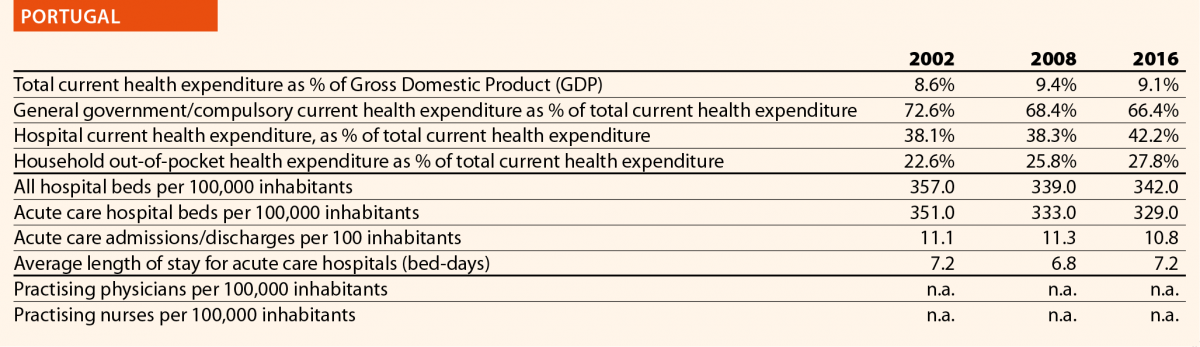

Mr Simon Vrhunec
HOPE Governor, Association of Health Institutions of Slovenia
Could you describe the last hospital and/or healthcare reforms implemented in your country in the past 5 years?
Slovenia is still among the countries in the EU that spend less than average on healthcare. If EU average health expenditure is 9.6% of GDP in 2017 (Health at a Glance: Europe 2018), in Slovenia it was only 8.0% of GDP the same year. In addition to the lack of financing resources Slovenia is facing also a lack of practicing doctors. In Slovenia there are 3 practising doctors per 1000 population while the EU average is 3.6 (Health at a Glance: Europe 2018). Only with practising nurses is the situation slightly better, because this figure is above the EU average in Slovenia.
A lack of resources in the Slovenian healthcare system results in long waiting times for specialist outpatient care. Some waiting times exceed a year and 40% of those who are waiting for a specialist outpatient service are waiting more than it is considered acceptable. Despite those facts, in politics there is still a very strong opinion that the main cause for long waiting times is corruption and not lack of resources, which is why no serious reform was implemented in the last five years. The only change of health law was to prevent (forbid) doctors to work for different employers and to decrease the validity of concessions from an indefinite term to 15 years.
Could you present two/three elements on the impact of such reforms on hospital and/or healthcare sectors that your organisation/country has identified?
Because the healthcare legislation was changed only one and a half year ago and the providers are still inside the three-year period to adopt the changes, there are no elements of the impact present. But the expectation is that restrictions for doctors to work on different locations will result in increasing waiting times and not the opposite.
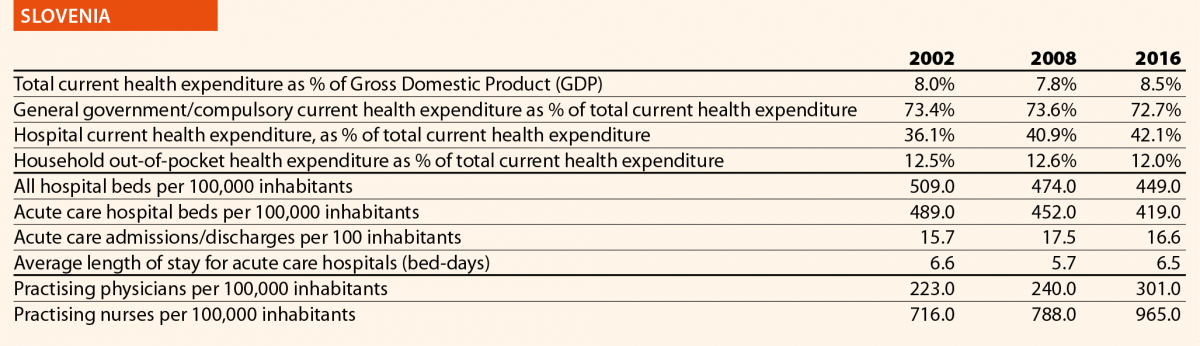

Mrs Sara Pupato Ferrari
HOPE Governor, Ministry of Health, Social Services and Equality
Could you describe the last hospital and/or healthcare reforms implemented in your country in the past 5 years?
Central procurement in the National Health System
On December 2001, the transfer of managerial processes regarding the National Healthcare System concluded with the decentralisation of decision-making centres to the 17 Autonomous Communities (CCAAs), the National Healthcare System itself and the National Health Management Institute (INGESA).
The decentralisation brought a very fragmented, opaque and, in some way, inefficient procurement market policies that required a rationalisation of the procurement system.
A Central Purchasing Unit dealing with all the National Healthcare System procurement procedures was considered a useful alternative for that, with the aim of:
During 2014, pharmaceutical products restricted to hospital use were declared as central procurement system products and INGESA was entrusted to develop the acquisition procedures. From 2015, most CCAAs (the exceptions being the Basque Country, Catalonia, Andalusia and the Canary Islands) have supported and participated in the emerging acquisition system replacing old punctual acquisition agreements and a Central Procurement Agency was implanted in INGESA aiming to get the support of the CCAAs. Despite the achievements, centralised procurement raised several issues and was partially used.
Strategic National Plan against Hepatitis C
Four years ago, the Strategic National Plan against Hepatitis C was established, and major achievements have occurred since then. An update of the Plan Against Hepatitis C has been approved by the National Health System Interterritorial Health Council and now new challenges have to be embraced to make this policy effective. The collaborative diagnoses and treatment of social groups that do not know whether or not they are infected also has to be achieved.
Could you present two/three elements on the impact of such reforms on hospital and/or healthcare sectors that your organisation/country has identified?
Central procurement in National Health System. Recently a centralised acquisitions digital tool has been introduced by the Ministry of Health, Consumers Affairs and Welfare. The tool is based on an Information Platform Collaborative Web that allows access to the participating agents to all the information related to centralised acquisition initiatives in an anonymous way.
It also incorporates a virtual forum so different agents can share problems and good practices on line easing collaboration in some issues such as catalogue definition, contract files development and criteria. Having elaborated a centralised acquisition products catalogue integrated in the platform, nearly 25,000 items structurally and hierarchically classified have been included. The catalogue structure will allow incorporation of medical services in the future. With this tool, a budget saving close to 500 Million Euros is anticipated in the coming years.
Hepatitis C strategy
Access to effective treatment could be considered fulfilled and to date, more than 117,000 patients can be considered cured in Spain.
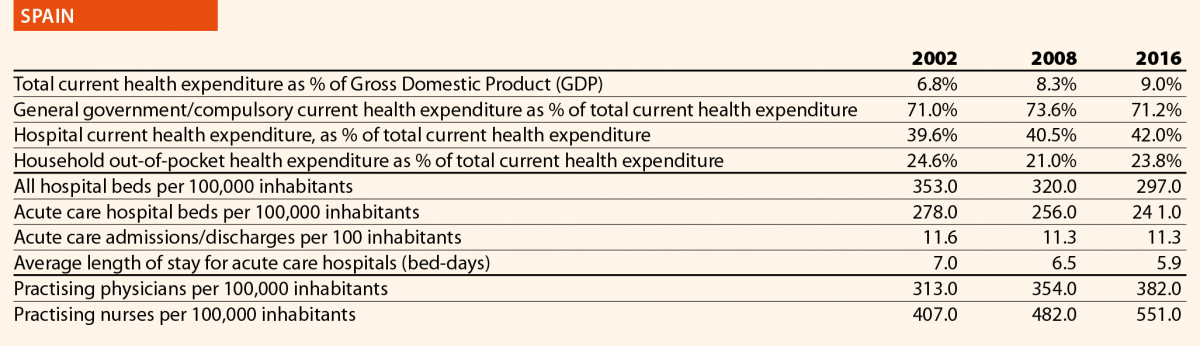

Mr Erik Svanfeldt
HOPE Governor, Swedish Association of Local Authorities and Regions (SALAR)
Could you describe the last hospital and/or healthcare reforms implemented in your country in the past 5 years?
In 2015, a new Patient Act came into force, which increased the opportunities for patients to choose healthcare provider – public or private – in outpatient care. In Sweden, the regions are responsible for the financing and provision of healthcare, and there are sometimes differences concerning what services they offer. In the past, the patients had limited possibilities to seek planned healthcare outside their home region, but since 2015 patients may also seek outpatient treatments in other parts of Sweden that are not included in their home region health basket.
In July 2018, a new decision-making process for national highly specialised healthcare was established. Sweden already had a system for coordination of very highly specialised healthcare, giving a national committee the right to concentrate certain services to just one or two hospitals in the entire country. But in order to improve quality and equality in healthcare, the National Board of Health and Welfare can now decide to concentrate specific healthcare services to up to five hospitals.
In parallel, work on level structuring and standardised care processes in cancer care is ongoing. Proposals have also been submitted on how to make the Swedish healthcare more efficient by improving community care. In 2018, a new law on cooperation at discharge from inpatient healthcare was implemented. The aim is to improve the care for those people who need both outpatient healthcare and the assistance of the municipal social services.
The regions have, one by one, enabled patients to access their own patient records online.
In 2013, a new legislation on health and medical care to foreigners who stay in Sweden without necessary permissions as implemented. The regions have to offer these people health and dental care who urgently require it.
The Swedish Parliament has recently decided to reform the training of doctors in 2020.
Could you present two/three elements on the impact of such reforms on hospital and/or healthcare sectors that your organisation/country has identified?
The new Patient Act, introduced in 2015, has had at least two noticeable effects. First of all, the new legislation, together with the EU Patient Mobility Directive from 2011, has widened Swedish patients’ access to European healthcare. Since 2015, the most generous region sets the bar concerning the patient’s rights to care and reimbursement. If one region offers a specific treatment, all Swedish patients have the right to receive this care on equal conditions also in another EU/EES member state.
Similarly, the legislation has allowed patients to make medical appointments and have video consultations via smartphone, personal computer or tablet across regional borders. A healthcare provider offering digital services can now establish in just one region and serve the entire country. In Sweden, each region decides on their own patient fees, mechanisms for paying the providers and remunerations, but because of these new cross-border digital services, there was a need for SALAR to adopt recommendations concerning the level of remuneration to the providers and uniform patient fees.
Concerning level structuring and standardised care processes in cancer care, the Swedish Government and SALAR in 2015–2018 implemented a national effort to shorten waiting times and reduce regional differences in cancer care by introducing cancer patient pathways. These pathways aim to shorten the time between a well-founded suspicion of cancer and the start of the first treatment. Another goal was to create a more equal and predictable care with increased quality and improved patient experience.
This national effort is monitored by following the waiting times and patient reported experience measures (PREMs). The measurements show high overall patient satisfaction with the cancer care and the national effort has also led to better communication within and between hospitals and regions. But so far, it has been difficult to influence waiting times, partly due to problems of lack of staff.
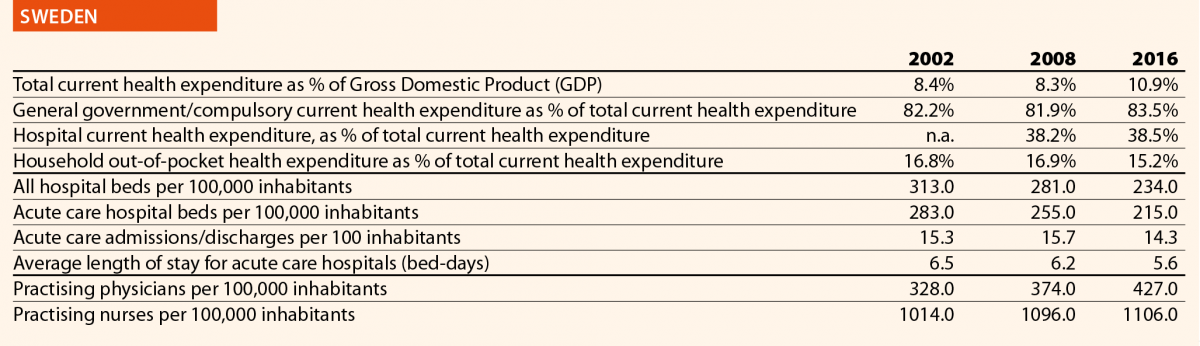

Mr Sander Gerritsen
HOPE Governor, Dutch Hospital Association
Could you describe the last hospital and/or healthcare reforms implemented in your country in the past 5 years?
A significant healthcare reform took place in 2006. It replaced the division between public and private insurance with one universal social health insurance. It also introduced managed competition as a driving mechanism. Although this reform was initiated over a decade ago and the principles of the new system have since then remained intact, its stepwise implementation continues to bring changes in the healthcare system.
Since 2015, there is one integrated reimbursement rate for hospital treatments. Until then costs were claimed partially by doctors and partially by hospitals. In the new system hospitals claim the entire costs for the treatment. It is up to them to distribute the money. This has led to new forms of cooperation between the doctors and the hospital management.
In 2015, the General Act on Exceptional Medical Expenses, which related to long-term care and nursing, was dismantled. The costs were running out of control and the government decided to act. Most of the tasks were decentralised and became the responsibility of the municipalities.
Could you present two/three elements on the impact of such reforms on hospital and/or healthcare sectors that your organisation/country has identified?
The first challenge concerns the monitoring of costs when there is a market-based system (production driven) while the demand for care is constantly growing.
The new financial structure has had its challenges. Doctors have formed separate medical specialist companies within the hospital structure. Hospitals have been struggling for longer to find the best ways to incorporate such companies within the hospitals. Nonetheless the Dutch Health Authority (NZa) concluded in 2018 that this reform has had positive effects on this cooperation (professionalisation, efficiency, shared responsibility).
Decentralisation of tasks does not simply lead to a reduction of costs. When you decentralise the long-term care without transferring enough funds, in the long run it might even have an adverse effect. One example is the closing of nursing homes, which led to a rise in the number of vulnerable elderly patients in hospitals.
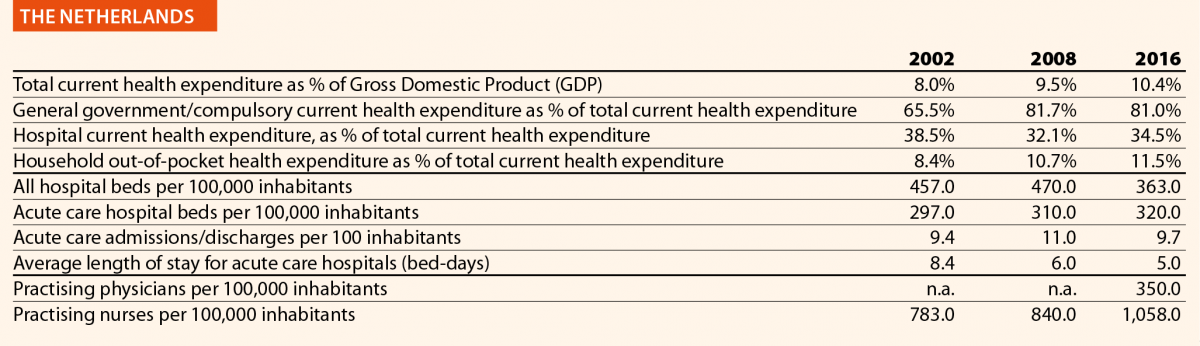

Mr Niall Dickson
HOPE Governor, NHS Confederation
Could you describe the last hospital and/or healthcare reforms implemented in your country in the past 5 years?
NHS Long-Term Plan
In January 2019, NHS England published the Long-Term Plan, setting out a strategy for the health service for the next ten years. It is a wide ranging and ambitious document, articulating a new service model with greater emphasis on primary and community care; increased emphasis on mental healthcare, prevention and health inequalities; a clear focus on quality and outcome improvement in areas where greater progress is seen as necessary; some workforce reforms; wholesale rebalancing of the NHS relationship with digital services and significant changes to the financial regime. The plan will be achieved by:
Integrated Care Systems
The NHS Five Year Forward View published in 2014 established Sustainability and Transformation Partnerships (STPs). STPs are now evolving into Integrated Care Systems (ICSs). Within the current legal framework, the NHS and its partners will be moving to create Integrated Care Systems everywhere by April 2021, building on the progress already made. ICSs bring together local organisations in a pragmatic and practical way to deliver the triple integration of primary and specialist care, physical and mental health services, and health with social care. They will have a key role in working with Local Authorities at place level, and through ICSs, commissioners will make shared decisions with providers on population health, service redesign and long-term plan implementation. ICS will deliver the aims set out in the NHS long-term plan.
Could you present two/three elements on the impact of such reforms on hospital and/or healthcare sectors that your organisation/country has identified?
Vanguards
Following three years of testing alternative models in the Five Year Forward View through integrated care Vanguards and Integrated Care Systems, we now know enough to commit to a series of community service redesigns everywhere. The Vanguards received less than one tenth of 1% of NHS funding, but made a positive impact on emergency admissions, and demonstrated the benefits of proactively identifying, assessing and supporting patients at higher risk to help them stay independent for longer.
Moving care closer to people’s homes and communities
Frimley STP’s North East Hampshire and Farnham Vanguard brought together primary, community, acute, mental health and social care service teams across the area to work as one. Closer working across traditional service boundaries helped to stimulate new services and initiatives, which support people, to manage their own health and care more effectively and to receive more care and support in their local community rather than in hospitals.
This includes the Time Out café in Aldershot for people with mental health problems, run across geographical and organisational boundaries to offer an alternative to A&E for those in crisis. Service users can access the café seven days a week with no appointment needed, to get support from staff, learn self-management skills and use community resources such as peer support and mental health and wellbeing advice. The scheme has resulted in a reported 33% reduction in acute psychiatric admissions. Residents’ feedback shows they particularly value being able to access help when they need it and that the café has helped to avoid further crisis, self-harm and unnecessary A&E visits.
Other examples include GPs in Erewash are supporting urgent care through an on-day service that has contributed to a 3.8% fall in non-elective admissions to hospital, despite overall rising A&E attendances. In Dudley, teams of doctors, nurses and other professionals have reduced the time people spend in hospital by the equivalent of 900 bed days in just over two years. Pharmacists, working alongside teams in local GPs’ surgeries, have helped reduced mortality from hypertension.
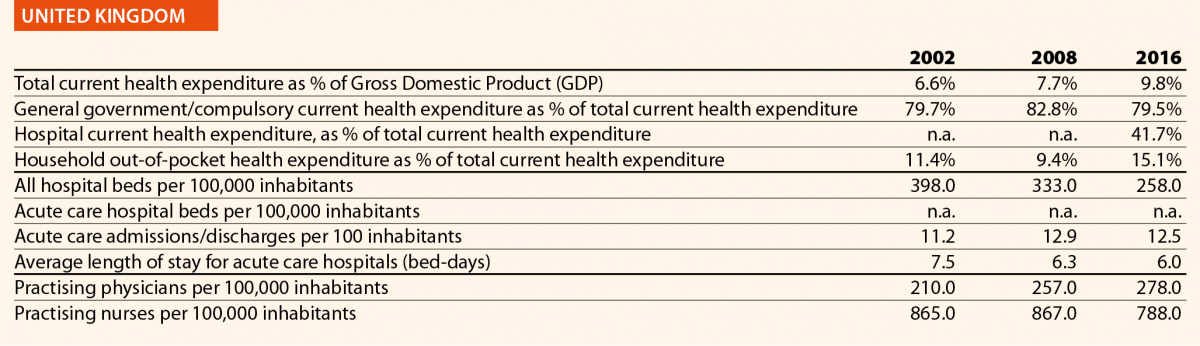
Further case studies and examples can be found here.
References
1 For more information on the Danish National Strategy for Personalised Medicine 2017-2020 click here.
2 For more information about the National Digital Health Strategy 2018-2022 click here.
3 The reply was provided in January 2019. The reform mentioned did not receive the approval on the Finnish Parliament and the Government resigned in March 2019. Ministério da Saúde (2018), Retrato da Saúde, Portugal. Available here.
4 Portaria n.º 71/2018. Available here.
5 For further information click on the following link.
6 Ministério da Saúde (2018), Retrato da Saúde, Portugal. Available here.
7 Estratégia Nacional para o Ecossistema de Informação de Saúde 2020 (ENESIS 2020). Available here.
8 Web-portal available here.
9 Mobile application MySNS available here.
10 Mobile application MySNS TEMPOS available here.
11 Mobile application MySNS CARTEIRA available here.
12 Decreto-Lei n.º61/2018 available here.
3rd September 2019
The European Society of Cardiology (ESC) Guidelines on supraventricular tachycardia have been published in the European Heart Journal, and on the ESC website.
Supraventricular tachycardia (SVT) refers to a heart rate above 100 beats per minute (normal resting heart rate is 60 to 100). It occurs when there is a fault with the electric system that controls the heart’s rhythm. SVTs are frequent arrhythmias, with a prevalence of approximately 0.2% in the general population. Women have a risk of developing SVT that is two times greater than men, while people 65 years or older have more than five times the risk of developing SVT than younger people.
SVTs usually start and stop suddenly. They arise in the atria of the heart and the conduction system above the ventricles, and are rarely life-threatening in the acute phase, unlike arrhythmias from the ventricles. However, most SVTs, if left untreated, are lifelong conditions that affect the heart’s function, increase the risk of stroke, and affect quality of life. Symptoms include palpitations, fatigue, light-headedness, chest discomfort, shortness of breath, and altered consciousness.
The guidelines provide treatment recommendations for all types of SVTs. Drug therapies for SVT have not fundamentally changed since the previous guidelines were published in 2003.
But Professor Josep Brugada, Chairperson of the guidelines Task Force and professor of medicine, University of Barcelona, Spain, said: “We do have more data on the potential benefits and risks associated with several drugs, and we know how to use them in a safer way. In addition, some new antiarrhythmic drugs are available.”
Antiarrhythmic drugs are useful for acute episodes. For long-term treatment these drugs are of limited value due to relatively low efficacy and related side-effects.
The main change in clinical practice over the last 16 years is related to the availability of more efficient and safe invasive methods for eradication of the arrhythmia through catheter ablation. This therapy uses heat or freezing to destroy the heart tissue causing the arrhythmia.
Professor Demosthenes Katritsis, Chairperson of the guidelines Task Force and director of the 3rd Cardiology Department, Hygeia Hospital, Athens, Greece, said: “Catheter ablation techniques and technology have evolved in a way that we can now offer this treatment modality to most of our patients with SVT.”
SVT is linked with a higher risk of complications during pregnancy, and specific recommendations are provided for pregnant women. All antiarrhythmic drugs should be avoided, if possible, within the first trimester of pregnancy. However, if necessary, some drugs may be used with caution during that period.
“Pregnant women with persistent arrhythmias that do not respond to drugs, or for whom drug therapy is contraindicated or not desirable, can now be treated with catheter ablation using new techniques that avoid exposing themselves or their baby to harmful levels of radiation,” said Prof Katritsis.
What should people do if they experience a fast heartbeat? “Always seek medical help and advice if you have a fast heartbeat,” said Prof Brugada. “If SVT is suspected, you should undergo electrophysiology studies with a view to catheter ablation, since several of the underlying conditions may have serious long-term side effects and inadvertently affect your wellbeing. Prevention of recurrences depends on the particular type of SVT, so ask your doctor for advice. Catheter ablation is safe and cures most SVTs.“