This website is intended for healthcare professionals only.
Take a look at a selection of our recent media coverage:
11th September 2019
Colorectal cancer is Europe’s second biggest cancer killer, claiming the lives of nearly 200,000 people across the continent each year. Current trends predict that the burden of colorectal cancer could increase by 12% by 2020, affecting 502,000 Europeans a year by 2020.1
Colorectal cancer screening acts as the most effective method in improving prognosis and preventing the disease. The heavily time-dependent nature of colorectal cancer means that there is a much higher chance of survival the earlier the cancer is detected, as early cancers have a survival rate of 90–95%.2 Despite the obvious threat posed by the disease and the possible advances screening programmes hold, access to effective screening programmes and treatment differs significantly across Europe.
Currently, eastern Europe is falling behind the west in the quality and efficacy of screening programmes and quality assurance mechanisms in these countries are not widespread. Low participation rates in screening programmes are frequent in eastern Europe, with many countries falling short of the 65% participation rate considered necessary by the European Commission. The inequality of access is highlighted when comparing the participation rates between Hungary and The Netherlands; whilst The Netherlands has attained a 72% participation rate, Hungary has a participation rate of just 0.6%. These inequalities act as an example for the insufficient efforts made by many governments across Europe to make colorectal cancer screening an accessible service and a key public health concern. European governments should be aiming to offer appropriate screening tests to the whole target population to detect colorectal cancer when it is at an early stage and before symptoms begin.
Despite colorectal cancer screening programmes attaining an established presence across different parts of Europe, many countries are yet to transition to the most effective and accurate screening methods. Presently, a number of European countries are still using the faecal occult blood test (gFOBT). The test relies on three samples that are tested for simple oxidation which can be compromised by the influence of dietary haemoglobin, leading to a number of false positives. The recently introduced faecal immunochemical test (FIT) is more advanced than the traditional gFOBT method. It is easier to perform, as it requires only a single stool sample to check for the presence of blood and has a simple collection device. Studies have revealed the increased sensitivity of FIT, resulting in increased cancer detection. FIT has also been viewed as a more acceptable test by members of the public due to the ease of collection.3
This method has also been demonstrated to have an increased participation uptake, and is therefore a more desirable alternative to the gFOBT method. In The Netherlands, for example, the participation rate for FIT was 12% higher than that for gFOBT.4 In a FIT-based screening program, colorectal cancers can be detected at an earlier stage than through symptoms. All FIT-positive participants are advised to undergo a colonoscopy, during which these cancers, as well as potentially cancerous polyps, will be identified. After diagnosis the optimal treatment-strategy will be chosen, of which one is endoscopic removal. An enhanced understanding of the genetic and epigenetic changes that are behind the formation of CRC aims to identify molecular markers for accurate and non-invasive screening tests. The addition of molecular markers to the FIT method could optimise screening accuracy in the future.
In the struggle to combat colorectal cancer, novel and effective treatments are urgently required. The surgical resection of tumours currently represents the best strategy to improve patient survival rates, however patients still have a high risk of developing metastases. Additionally, chemotherapy is not optimally beneficial for all patients who are diagnosed with advanced colorectal cancer due to factors like poor efficacy, drug resistance and severe side effects.5 In recent years it has become clear that not all colorectal cancers are the same, and that the molecular characteristics of the tumours should be taken into account. Molecular differences mean that a ‘one-size-fits-all’ approach to treatment is not optimal, leading to an increased interest in a personalised method of treatment.6 Personalised colorectal cancer treatment utilises information about a person’s genes, proteins and environment to prevent, diagnose and treat the disease. The ability to use molecular screening to characterise tumours and target patients who are likely to benefit from personalised treatment, holds great potential for positive patient outcomes. Further research into this mode of tailored treatment could have a transformative effect on the concerning upward trend of colorectal cancer treatment modalities and reduced mortality rates.
Aligning with the move towards more personalised forms of treatment, recent developments in artificial intelligence (AI) may also aid the detection and treatment of tumours. The advent of AI represents an exciting forefront in cancer prevention. An endoscopic system powered by AI has been shown to automatically identify colorectal adenomas and early cancers during colonoscopy. The computer-aided diagnostic system might also use endoscopic, or a more detailed endocytoscopic imaging to analyse the polyp, comparing it to other images, allowing prediction of lesion pathology in less than a second.7 The use of AI holds the possibility of aiding the early identification of potential cancerous adenomas, helping to reduce the incidence and mortality of colorectal cancer.
The early detection of colorectal cancer or potentially cancerous polyps is the most vital action in reducing colorectal cancer incidence and mortality. However, a healthy lifestyle can also act as an integral measure. Alarming figures have shown that European colorectal cancer rates in young adults is increasing by 6% per year, which has been linked to poor diets, sedentary lifestyles and obesity, with over half of he EU population being considered overweight.8,9 The promotion of healthy lifestyles, reduced alcohol consumption and a reduction of the European population’s meat consumption should become a strong focus of European policy. An enhanced public understanding of healthy lifestyle options is an essential measure in mitigating the threat of colorectal cancer.
The public’s participation in screening programmes also plays a crucial role in the success of early detection. Responding to invitations and completing the at-home tests that are available across many parts of Europe could greatly reduce the incidence and mortality rates that are attributable to colorectal cancer. Health professionals can also act as proactive figures in prevention and early detection. Research has suggested that the lack of colorectal cancer screening recommendations made by a doctor is a key barrier to screening uptake.10 As one of the most accessible authorities on health matters to the general public, healthcare professionals should be broaching the subject of screening with eligible adults and extolling the benefits of colorectal cancer screening.
The education of members of the public about the advantageous aspects of colorectal cancer screening by trusted healthcare professionals will also help expel the negative connotations associated with the screening process. The initial gFOBT or FIT screening methods are largely painless and various initiatives are being undertaken across Europe to improve the quality of colonoscopies and reduce its burden. In order to improve detection rates, colorectal cancer screening must become a normative and essential aspect of healthcare across Europe that is dissociated from feelings of fear and embarrassment.
Ultimately, the most effective and vital component in the battle against colorectal cancer is detecting its presence as early as possible, as colorectal cancer symptoms only become prevalent in the later stages of the disease. A harmonisation of screening programmes and increased participation rates needs to become a key priority for governments across Europe.
Professor Evelien Dekker, colorectal cancer expert, is a member of the European Gastroenterology (UEG) Public Affairs Committee. Professor Dekker’s expertise derives partly from the Netherlands, in which pilot-studies and implementation studies were conducted to investigate the most effective ways of organising the programme and encouraging enrolment.
The Netherlands currently has the highest rate of participation in colorectal cancer screening programmes in Europe. UEG is a professional non-profit organisation encompassing all the leading European medical specialist and national societies focussing on digestive health. UEG aims to reduce the burden of digestive diseases through improved disease prevention, diagnosis, cure and raised awareness of their importance.
References
1 International Agency for Research on Cancer (IARC).
2 Arnold M et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66(4):683–91.
3 Mousavinezhad M et al. The effectiveness of FOBT vs. FIT: A meta-analysis on colorectal cancer screening test. Med J Islam Repub Iran 2016;30:366.
4 United European Gastroenterology. 2016. One in four cases of CRC diagnosed within two years of a negative screening result. www.ueg.eu/press/releases/ueg-press-release/article/one-in-four-…. (accessed August 2019).
5 United European Gastroenterology. 2016. Experimental models of colorectal cancer. www.ueg.eu/education/latest-news/article/article/experimental-mo…. (accessed August 2019).
6 Moorcraft SY, Smyth E, Cunningham D. The role of personalized medicine in metastatic colorectal cancer: an evolving landscape. Therap Adv Gastroenterol 2013;6(5): 381–95.
7 United European Gastroenterology. 2017. Artificial intelligence: is this the future of early colorectal cancer detection? www.ueg.eu/press/releases/ueg-press-release/article/ueg-week-art…. (accessed August 2019).
8 United European Gastroenterology. 2018. European colorectal cancer rates in young adults increasing by 6% per year. www.ueg.eu/press/releases/ueg-press-release/article/ueg-week-eur…. (accessed August 2019).
9 Eurostat: Statistics Explained. 2014. Overweight and obesity- BMI statistics. https://ec.europa.eu/eurostat/statistics-explained/index.php/Overweight_…. (Accessed 6 February 2019).
10 United European Gastroenterology. 2014. Family doctor intervention is crucial in the fight against Europe’s second biggest cancer killer. www.ueg.eu/press/releases/ueg-press-release/article/family-docto…. (accessed August 2019).
The majority of cytotoxic drugs are administered as sterile injections or infusions, which means asepsis must be maintained during the preparation and administration of chemotherapy, particularly because many patients are immunocompromised. Protecting healthcare staff from occupational exposure, ensuring sterility of parenteral chemotherapy and complex clinical management issues all combine to present a multidimensional challenge to pharmacy staff and specialist chemotherapy nurses.
This opinion piece considers the different technologies used for the preparation of cytotoxic injections and infusions: pharmaceutical isolators, Class II cytotoxic cabinets (also erroneously referred to as vertical laminar flow cabinets), and closed system transfer devices (CSTDs). The attributes and limitations of each technology are considered in terms of the maintenance of infusion/injection sterility (protection of the product from the environment), containment of the cytotoxic medicine (protection of staff from the product), conserving the pharmaceutical integrity of the product and issues around the incorporation of these technologies into oncology pharmacy practice.
For simple aseptic manipulations involving reconstitution of vials, withdrawal of liquid and dilution to prepare pre-filled syringes or infusion bags, both the class II cabinet and the isolator offer critical zone environments corresponding to EUGMP Grade A. This is conditional upon maintenance, monitoring, testing and validation for both types of device, the details of which are available elsewhere. In theory at least, the Class II cabinet is more vulnerable to changes in airflow in the vicinity of the cabinet caused, for example, by personnel moving around in the aseptic suite.1 This can disrupt the laminar airflow at the open face and draw in air from outside the critical zone. Conversely, a pharmaceutical isolator should, if properly maintained and validated, sustain the Grade A environment against such challenges. The isolator offers potential design advantages in that materials are introduced into the critical zone via flushed hatch systems with interlocking doors. This enables the implementation of a time delay between the closing of the outer hatch door and opening of the inner hatch door to the Grade A work zone, which, in turn, enforces a minimum contact time for surface disinfectants sprayed, or wiped onto the surface of in-bound materials or packaging to exert a bactericidal effect. Isolators, either connected in series or used singly, offer the potential for gaseous sterilisation of consumables introduced into the isolator prior to aseptic manipulation. This approach, which is best applied to batch-scale preparation for applications such as dose-banding, requires rigorous validation and systems to ensure there is no ingress of sterilising gas (usually powerful oxidising agents) into the product. The main disadvantage of isolators when compared with Class II cabinets is that they are more difficult to clean and to sanitise internal surfaces.2
There has been much debate over the use of positive- or negative-pressure isolators for cytotoxic manipulation; the former, in theory, is more likely to maintain the aseptic environment in the event of a leak in the isolator, but the latter provides a higher level of operator protection in the same scenario. Guidance based on a limited study conducted by the UK Health and Safety Executive was inconclusive and suggested either positive or negative isolators were acceptable providing they were properly maintained and operated.3
Unlike isolators and Class II cabinets, which provide an aseptic environment and containment, CSTDs provide a closed, sterile fluid path for aseptic manipulation, with either an expansion chamber or a filtration system to permit displacement of air by liquid. In most cases, the CSTD accesses the drug vial or the infusion bag via a spike or retractable needle system. When deployed in a non-aseptic environment there must be the potential risk of microbial inoculation into the vial or the infusion. In an uncontrolled environment the manipulation of CSTD connectors and docking devices may also carry a risk of microbiological contamination, even when sophisticated valve systems are used to mitigate this risk. Additionally, the integrity of the seal between the drug vial or infusion bag septum and the CSTD spike or needle is not only dependent in the design of the CSTD, but also on the material and design of the vial septum and this can vary significantly between manufacturers.
In recent years, CSTDs have been used for drug reconstitution in clinical areas outside the pharmacy, particularly for monoclonal antibodies. While this practice is undoubtedly an improvement over the use of open systems in an uncontrolled environment, it should not be assumed that product sterility is automatically guaranteed. There is huge variation in the environmental quality of clinical areas in oncology units, variation in cleaning and sanitisation practices, differences in staff training and competency assessment and, subsequently, the potential for significant variation in the bioburden that challenges the integrity of the CSTD in different centres. In the opinion of the author, the use of CSTDs in this type of environment requires careful validation and control for each individual area in which infusions are prepared to ensure sterility is not compromised. Closed systems are increasingly deployed in infusion/injection administration sets to minimise the exposure of chemotherapy nurses to cytotoxic drugs in outpatient clinics. There is currently much debate concerning whether pharmacy should provide infusion bags and syringes of cytotoxic drugs with CSTD devices already fitted. The aim is to avoid compromising sterility or risking cytotoxic contamination when nurses spike infusion bags or connect syringes, but in solving these issues other problems around product integrity are created. Progress on these matters will require much more ‘joined-up’ thinking between nursing and pharmacy staff.
In the pharmacy setting, mechanical ventilation in the form of Class II cytotoxic cabinets and isolators are at the top end of the controls designed to protect staff from occupational exposure to cytotoxic drugs. The hierarchy beneath this includes staff training, safe systems of work, personal protective equipment and environmental monitoring. Both Class II cabinets and pharmaceutical isolators discharge air outside of the isolator through one or two HEPA filters, and ideally to the outside of the building via fan-assisted external ducting. As with product protection, above, the open face of the Class II cabinet presents a potential weakness in that disturbance of the air flow in the ‘protective curtain’ of air directed vertically from the top of the open face into the plenum at the base of the work area might permit air currents to drift from the cabinet to the outside. The author has seen smoke tube experiments where a combination of overloading the cabinet with equipment (syringe filling pump) and movement in the aseptic room in front of the cabinet have resulted in trails of smoke tracking along the operator’s arms, through the protective curtain and into the proximity of the operators face.
Isolators provide a physical barrier between the cytotoxic manipulation area and the operator. After initial resistance from UK pharmacy staff in the 1980s, most technicians and pharmacy assistants prefer isolators because they ‘feel safer’. There may, however, be an element of a ‘false sense of security’ about this. In the case of a positive pressure isolator, leaks in the isolator chamber, gloves or sleeves, exhaust HEPA filters or the external ducting could result in contaminated air being pumped into the outside environment. As with Class II cabinets, isolators (whether operated under positive or negative pressure) must be validated, monitored, tested and fully maintained to work effectively.
A less obvious problem with isolators relates to the fact they are containment devices, and also difficult to clean and remove cytotoxic drug residues.2 This means that the inside of isolators and transfer hatches can become contaminated with cytotoxics, even when regular cleaning routines are deployed. This issue has been demonstrated in the ‘real-life’ setting4 where cytotoxic contamination was not only found on wipe samples from the inside of the isolator, but also on the external surfaces of pre-filled syringes and infusion bags passed out of the isolator and into the aseptic room. When these are then taken to clinical areas for administration to patients, the cytotoxic contamination is transferred with them, potentially contaminating clinic and patient areas and exposing nursing staff, non-cancer patients and patient carers to risk. It would therefore be erroneous to think of isolators as a ‘single and final solution’ for reducing cytotoxic contamination in the pharmacy and the clinic.
CSTDs are designed to contain cytotoxic residues and aerosols within the device itself. There has been debate over which CSTDs on the market are ‘genuine’ closed systems. Manufacturers of devices with air-displacement chamber technology argue that devices where displaced air is exhausted through hydrophobic filters and activated charcoal are not closed systems amid concern that not all cytotoxic molecules are trapped by the filter system and that repeated use may saturate the filter. Reference to studies published on these devices tends to show that they all reduce contamination on workplace surfaces when tested in the field using cytotoxic drugs, irrespective of the CSTD design, albeit with some variation in effectiveness.5,6 In one study,4 the CSTD significantly reduced cytotoxic contamination on the external surfaces of pre-filled syringes and infusion bags leaving the isolator when compared with standard needle and venting pin techniques. Even with these encouraging findings, CSTDs should not be considered a ‘panacea’ for eliminating cytotoxic contamination and some devices exhibit residual droplets of liquid on docking valves after disconnection. Also, contamination may arise from multiple sources and some of these are not mitigated by the use of CSTD technology, for example contamination on the outside of drug vials. The cost of implementing CSTDs may also prove challenging.
There seems to be a developing argument for combining the use of CSTDs and isolators, with the intention of reducing cytotoxic contamination inside the isolator and minimising the transfer of cytotoxic residues to the outer surface of syringes and infusion bags leaving the isolator. The key question then would be whether this measure, when combined with the use of closed-system infusion administration sets, can reduce cytotoxic contamination in clinical areas and reduce the occupational exposure risk to nursing and clinic staff. There is a clear need for research in both pharmacy and clinical areas to evaluate this approach. The paucity of evidence on the effectiveness of CSTDs was highlighted in a Cochrane Review7 commissioned by the UK Oncology Nursing Society. Some experts in the field consider this review to be poorly conducted and unhelpful,8,9 but it does serve to highlight the need for well-designed studies to evaluate new technologies or combinations of technologies.
The current interest in robotic chemotherapy compounding systems may present another opportunity for combining isolator/Class II cabinet and CSTD technology. With the cleaning and decontamination issues presented by complex robotic systems, this combination offers the potential for environment and product protection together with reduced cross-contamination between batches of different products.
Regulatory controls and microbiological considerations restrict the re-use of part-used vials of chemotherapy drugs. This results in considerable drug wastage which is unacceptable in the challenging economic conditions of modern cancer care. The use of CSTDs has been proposed as a potential option to effectively replace a multiple needle-entry vial septum with a syringe docking device to maintain vial integrity and permit multiple or extended use.10 However, as discussed above, there is still the integrity of the seal between the CSTD spike or needle and the vial septum over prolonged time-periods to consider. Use of CSTDs in this context would need extensive microbiological validation and also an assessment of materials leaching from the device fluid path when in prolonged contact with cytotoxic drug infusions, some of which are formulated with aggressive co-solvent systems which could attack plastic and metal components of the device.
It seems likely that optimal cytotoxic containment and maintenance of infusion sterility requires a combination of current technologies. The implementation of CSTDs in pharmaceutical compounding units when used in combination with isolators, and their use in clinics for chemotherapy administration, may be the way forward. This would require a joined-up approach by pharmacy and nursing staff, and careful evaluation in terms of cost and benefit.
References
1 The Cytotoxics Handbook, 4th edition. Editors Allwood, Stanley and Wright: Chapter 2, Facilities. Radcliffe Medical Press 2002; Abingdon, UK. ISBN 1 85775 504 9.
2 Roberts S et al. Studies on the decontamination of surfaces exposed to cytotoxic drugs in chemotherapy workstations. J Oncol Pharm Pract 2006;12:95–104.
3 Mason H. Cytotoxic drug exposure in two pharmacies using positive or negative pressurised enclosures for the formulation of cytotoxic drugs. Health and Safety Executive 2005, Report No. HEF/01/01 HSL Sheffield UK.
4 Vyas N et al. Evaluation of a closed-system cytotoxic drug transfer device in a pharmaceutical isolator. J Oncol Pharm Pract 2016;22(1):10–19.
5 Clark BA, Sessink PJM. Use of a closed system drug – transfer device eliminates surface contamination with antineoplastic agents. J Oncol Pharm Pract 2013;19(2):99–104.
6 Bartel SB, Tyler TG, Power LA. Multicentre evaluation of a new closed-system drug transfer device in reducing surface contamination by antineoplastics. Am J Health-Syst Pharm 2018;75(4):199–211.
7 Gurusamy KS et al. Closed-system drug-transfer devices in addition to safe handling of hazardous drugs versus safe handling alone for reducing exposure to infusional hazardous drugs in healthcare staff. Cochrane Database Syst Rev 2018;Mar(3):CD012860.
8 Connor T. Evidence of CSTD benefits: A rebuttal. Cleanrooms Compounding 2018;S6–S12.
9 McDiamid MA et al. Published review of closed-system drug transfer devices: Limitations and implications. Am J Health-Syst Pharm 2018;75:874–7.
10 Gilbar PJ et al. How can the use of closed system transfer devices to facilitate sharing of drug vials be optimised to achieve maximum cost savings? J Oncol Pharm Pract 2019;25(1):205–9.
Note
As an opinion piece, this article is not extensively referenced. If additional reference sources are required, please contact the author.
The dosing of chemotherapy agents involves a delicate balance between the desired efficacy and the drug’s acceptable toxicity. Traditionally, the doses of anticancer drugs were calculated according to body weight or surface area, but in 2018, the National Institute for Health and Care Excellence (NICE) released a position statement supporting dose standardisation, or dose banding, for intravenous chemotherapy drugs for adults with cancer. This standardisation and optimisation of doses in oncology brings benefits to hospitals, as well as health care providers and patients.
The objective of having standardised doses in the treatment of cancer is to facilitate the preparation of intravenous chemotherapy agents within hospital pharmacy aseptic units, and also the administration in the oncology ward. The chemotherapy schedule for a patient with cancer is often complex and involves multiple drugs and dose adjustments as part of a regimen that is tailored to each individual case. With dose standardisation, the doses of intravenous anticancer drugs are approximated to pre-determined standard doses and clustered in dose ranges or bands, which can significantly reduce preparation time, mitigate the risk of calculation errors, and reduce waste resulting from left-over drugs.1
The chemotherapy drugs purchased in bulk by a particular hospital can then be standardised to match the recommended pre-defined dose bands and prepared as single-serve doses in advance, provided these drugs show long-term physicochemical stability after compounding (that is, greater than 30 days). Alternatively, pre-compounded products can be acquired.
This dose standardisation process involves, in general; the approval of basic practices for the definition of dose bands by the local oncology and hematology departments; the approval of national or regional dose banding tables by local formulary committees; the prescription and dispensing of the approved drugs according to the doses listed in these tables; and the establishment of uniform practices for the purchase of ready-to-administer products.1
The role of the pharmacist in the administration of chemotherapy agents is critical, ranging from the verification of the prescription to the preparation of the drug(s) to be administered. The verification of prescriptions, which might involve a specific number and type of checks for each prescription, and the reconciliation of systemic anticancer agents, must be carried out by accredited professionals, according to standards defined by the British Oncology Pharmacy Association (BOPA).2
Prescription errors can be particularly dangerous in this setting. An analysis of prescriptions for chemotherapy agents against BOPA standards in England, Scotland and Wales identified errors in 2.3% of prescriptions during the mandatory prescription verification process, which could potentially result in medical intervention or hospitalisation, or even serious injury or death. In addition to prescription verification, pharmacists are responsible for reviewing all the medicines taken by a patient who is about to initiate chemotherapy in order to minimise the risk of adverse effects of the treatment such as toxicities or reduced efficacy owing to presence of comorbidities or the use of concomitant and/or alternative medicines that can interact with the conventional anticancer agents. Together with nurses trained in oncology, pharmacists can also provide counselling to patients and follow up on the use of any medicines, ensuring adherence to the treatment.2
Given the toxicity of these drugs and the significant investment in time and money from the institutions and staff, it is of utmost importance that all health care professionals involved in chemotherapy patients with cancer adhere to the standards for dose banding defined by their organisations. Regular quality audits should be conducted at all levels of involvement, and any deficiencies should be promptly communicated and corrected.2
Chemotherapy dose bands have been gradually implemented at several hospital trusts in England for the past ten years, although at variable pace. However, some level of heterogeneity has been observed in terms of processes used for dose banding and the drugs included in the bands.
In Scotland, where dose standardisation is now established, 60–70% of all chemotherapy agents are currently being administered according to pre-specified dose bands.1
In order to promote the adoption of dose banding for several intravenous anticancer agents, National Health Service (NHS) England has formed Medicines Optimisation and Chemotherapy Clinical Reference Groups with the aim to uniformise prescription practices across the entire region based on previously approved dose bands. The implementation efforts of these reference groups are being encouraged by NHS England via a Commissioning for Quality and Innovation initiative (CQUIN) scheme, which releases funding upon demonstration of improvements in quality from the participating trusts.1
Currently, there are pre-determined dose bands for 54 chemotherapy agents in England. NHS England’s Medicines Optimisation Intelligence Group is responsible for collecting data to support this implementation, and specific measurement tools and recommendations have been developed by the Chemotherapy Dose Standardisation Steering Group to measure the impact of this initiative. NICE is closely collaborating with NHS England to widen the implementation of dose standardisation for chemotherapy drugs, providing specific guidance on drug sourcing and supply, as well as contracting and tendering. In addition, NICE provides recommendations for the identification of waste during the preparation process, and for the measurement of the impact of dose banding on the patients’ experience with their treatment, as well as on staff satisfaction and the financial impact on Trusts.1
The benefits of chemotherapy dose standardisation are obvious, most notably the time-saving and cost-reducing benefits. In England, the costs incurred by the NHS with chemotherapy amount to approximately £1.5 billion, of which 80% represent anticancer medicines. In addition, these costs seem to grow by approximately 8% every year, which significantly contributes to the financial burden of the health system. With the implementation of discrete dose bands, the administered doses of conventional chemotherapy agents are actually about 6% of the calculated dose for the patient; for biological agents, which are traditionally much more expensive, it is approximately 10%.1
An analysis of costs and parenteral chemotherapy drug use following dose standardisation in a tertiary oncology centre in England showed a reduction in approximately £100,000 per month on 17 dose-banded drugs, despite an increase in the number of prescribed doses during the same period of time. These encouraging results were accompanied by a reduction of approximately 10% in the total workload associated with drug compounding, ultimately increasing the capacity and productivity of the centre’s aseptic compounding unit.3
Dose banding can also reduce patients’ waiting times because the ready-to-use drugs can be administered on any day that fits their schedules. Moreover, this practice allows patients to receive their treatment at facilities closer to their residence, given that no special compounding units are required for drug preparation. From the perspective of health care providers, dose banding results in reductions in the time spent with drug preparation and minimises dose calculation errors. Dose banding also prevents time-consuming changes in prescriptions and allows for a rapid dispensation through the use of pre-prepared doses. Financial efficiency can be further improved by outsourcing standardised pre-filled bags of chemotherapy agents for infusion. For commissioners, the uniformisation of doses at the national or regional level contributes to reduced costs arising from the reuse of doses that are not used due to changes in doses during treatment or due to cancellation of the treatment, and from the reduction in incomplete vial usage during the preparation process.1
In addition to these proven benefits, the available evidence suggests that the use of dose bands does not seem to have a negative impact on the toxicity associated with chemotherapy drugs or on clinical outcomes for patients.1
A retrospective study conducted in France in 2012 to compare the pharmacokinetic profiles of chemotherapy drugs, administered at regular fixed doses and as dose bands, showed no differences in drug exposure between the two dosing approaches.4 Another study in England showed the feasibility of the dose banding strategy for five anticancer drugs in paediatric patients with ages ranging from 1 month to 18 years, based on pharmacokinetic parameters.5
The standardisation of doses of intravenous cytotoxic chemotherapy agents was initially proposed to improve pharmacy capacity and reduce medication errors and wastage. However, further optimisation of the administration of anticancer drugs can potentially contribute to a more efficient oncology unit. In addition to dose bandings, the use of solvents, volume and labelling of chemotherapy agents can also be subject to uniformisation, which can potentially minimise the risks posed by these toxic drugs to both patients and staff. In the future, a partial or full automation of the drug preparation process may represent an advancement in terms of improvements in drug management since the number of patients diagnosed with cancer continues to increase every year. Formal evaluation of the feasibility, consistency, quality control and assurance of validated dose banding procedures in routine practice are also needed in order to demonstrate a reduction of the financial pressure placed on health systems due to non-standardised dosing.
References
1 National Institute for Health and Care Excellence. Chemotherapy dose standardization. February 2018. www.nice.org.uk/advice/ktt22 (accessed February 2019).
2 British Oncology Pharmacy Association. Medicines Optimisation, Safety and Clinical Pharmacy Workforce Plan. January 2015. www.bopawebsite.org/sites/default/files/publications/Clinical_pharmacy_w… (accessed February 2019).
3 Chatelut E et al. Dose banding as an alternative to body surface area-based dosing of chemotherapeutic agents. Br J Cancer 2012;107(7):1100–6.
4 Finch M, Masters N. Implications of parenteral chemotherapy dose standardisation in a tertiary oncology centre. J Oncol Pharm Pract 2018:1078155218812943.
5 White-Koning M. Investigating the potential impact of dose banding for systemic anti-cancer therapy in the paediatric setting based on pharmacokinetic evidence. Eur J Cancer 2018;91:56–67.
In December 2018, the National Institute for Health and Care Excellence (NICE) published its long-awaited update to the 2010 guideline on chronic obstructive pulmonary disease (COPD) in over 16-year-olds. A long time had elapsed between the update and previous guideline, and other guidance, such as that produced (and updated more frequently) by the Global initiative for chronic Obstructive Lung Disease (GOLD), has been useful in the interim to individualise treatment for patients. This article will outline the significant changes in the NICE guidance.
COPD is a progressive long-term condition and leading cause of death and disability; it has an estimated cost to the National Health Service over £800 million pounds per year. The mortality rate in England is roughly 23,000 deaths each year (around one person every 20 minutes).1
COPD is associated with current or history of smoking and/or biomass fuel/noxious particle exposure and usually affecting people over the age of 35 years (and often diagnosed in their 50’s). The ageing population is living for longer, often with a poorer quality of life, which itself presents a challenge to the healthcare system. Current generations may have started smoking from a younger age than previous, resulting in earlier onset of poor health. Prevalence is associated with geographical levels of deprivation and is increasing; many millions remain undiagnosed and by 2020, COPD will be the third leading cause of death globally.1,2
COPD is characterised by breathlessness and cough. Patients will typically experience exacerbations (some patients more so) which negatively impacts disease progression, rates of hospitalisation and readmission and health status. The number of exacerbations in the year prior is the strongest predictor of a patient’s future exacerbation frequency.3 The rate of lung function decline is faster in the earlier stages of the disease, which can be modified by treatment.1
The new guideline specifically acknowledges the need for a secure diagnosis, made using signs and symptoms and confirmed through spirometry by appropriately trained healthcare workers (who have up to date skills and are competent in interpreting results). This includes noting exacerbation history, excluding conditions such as asthma/cancer and consideration of alternative diagnoses such as alpha-1 antitrypsin disease. Diagnosis should also be considered in symptomatic individuals with normal spirometry. Oral steroids should not be used for reversibility testing or to predict response to inhaled corticosteroid (ICS) in individuals.2
Key opinion leaders and emerging research suggest that there are disease phenotypes (frequent exacerbator or persistent symptomatic patient).3 However, the place of inhaled triple therapy (where ‘triple therapy’ refers to use of long-acting beta agonist (LABA), long-acting anti-muscarinic agent (LAMA) and ICS together), asthma–COPD overlap management, role of eosinophils3 and role of macrolide antibiotics in disease management are also important considerations (which the guideline attempts to address) but require longer term data to further inform future practice. Updates in this guideline have been made on the following:7
These supplement the existing recommendations on:
NICE has produced a visual summary alongside the guidance, covering non-pharmacological management and use of inhaled therapies (Figure 1). They also published concurrent antimicrobial prescribing guidance for acute exacerbations of COPD (December 2018).
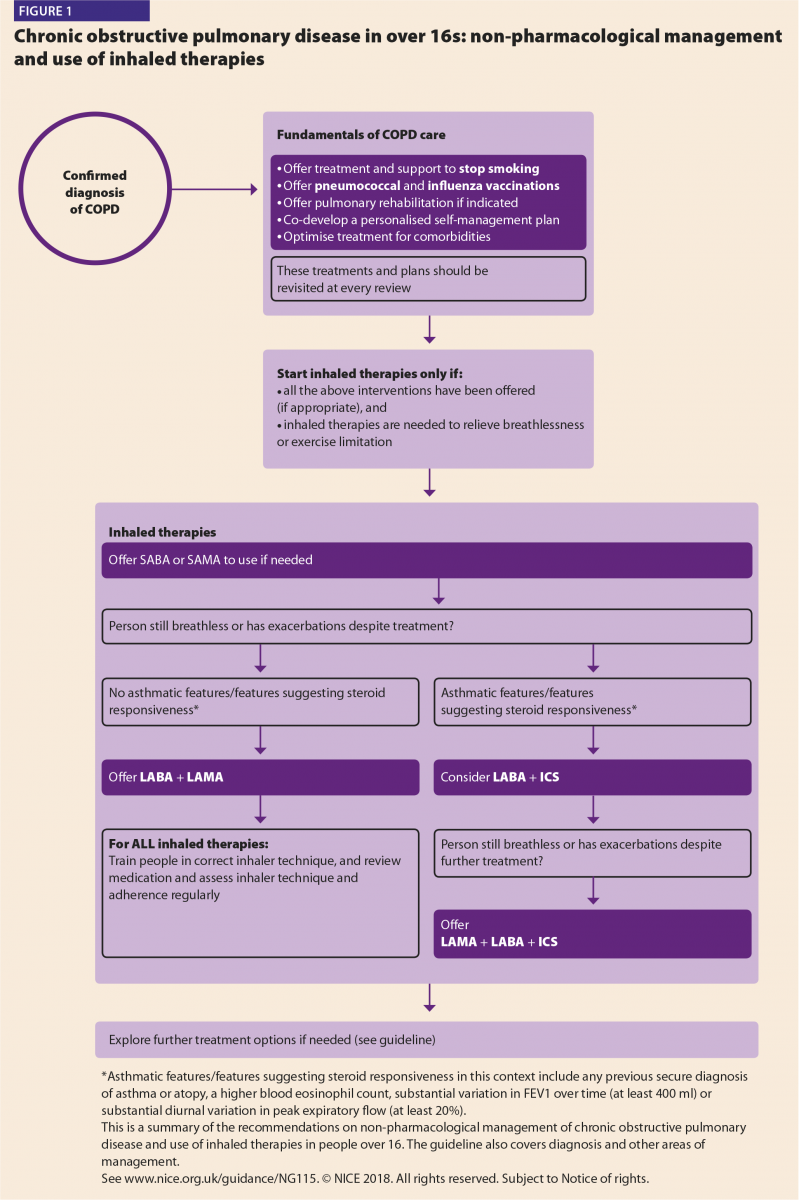
The 2010 guidance inevitably steered patients towards triple therapy (with LABA, LAMA and ICS) with a greater emphasis on FEV1 (forced expiratory volume in one second) value to guide this. Early therapy started with a short-acting bronchodilator progressing to a single long-acting bronchodilator (evidence subsequently showed superiority of LAMA over LABA use first line, especially in moderate–severe patients due to lower incidence of exacerbations and adverse effects with LAMA);4,5 stepping up to dual long acting agent and then addition of an ICS (a suggestion which was based on expert opinion).
It is now apparent that as a result, some patients were treated with ICS who may not have warranted it. The new guidance still initiates inhaled therapy with a short-acting bronchodilator but progresses to long-acting
dual therapy.
The new guidance does not rely on FEV1 to adjust therapy. One key change, stepping up after short acting bronchodilator, is the initiation of dual bronchodilator (LABA+LAMA) in preference to a single long acting agent. For patients exhibiting asthmatic features, the dual therapy would be initiated with ICS and LABA rather than LABA/LAMA [‘Where asthmatic features/features suggesting steroid responsiveness in this context include any previous secure diagnosis of asthma or atopy, a higher blood eosinophil count, substantial variation in FEV1 over time (at least 400ml) or substantial diurnal variation in peak expiratory flow (at least 20%)’].2
Patients using long-acting therapy outside of the December 2018 recommendations can continue on current treatment and change after review with a healthcare professional (HCP), when both agree is appropriate.
NICE is undertaking a separate review on the role of triple therapy in COPD (which was out of scope of the initial guidance update). At time of writing this article, the draft is undergoing public consultation and final guidance is scheduled to be published in Summer 2019. The draft 2019 guidance showed the proposed place of triple therapy in patients who remain breathless +/- exacerbate despite all other interventions being optimised (noting consideration of a three-month trial in those without asthmatic features, reverting if ineffective).6
Management of COPD can be broadly divided into two areas – non-pharmacological and pharmacological.
Non-pharmacological
These measures should be optimised alongside any pharmacological intervention and before escalation of therapy:
Pharmacological
Other considerations
NICE quality standards are a concise set of prioritised statements designed to drive measurable improvements in the three dimensions of quality – patient safety, patient experience and clinical effectiveness – for a particular area of health or care. They are derived from highquality guidance from, for example, NICE or other sources accredited by NICE. The COPD quality standard (last updated in 2016), in conjunction with the guidance, should contribute to the improvements outlined in the following three outcomes frameworks published by the Department of Health:20
NHS Outcomes Framework 2015–16
Reducing premature mortality, enhancing quality of life for people with long term conditions, helping people to recover from episodes of ill health or following injury and ensuring that people have a positive experience of care.
Public Health Outcomes Framework 2013–16
Health improvement/healthcare public health and preventing premature mortality.
Adult Social Care Outcomes Framework 2015–16
Delaying and reducing the need for care and support.
The quality standard is expected to contribute to improvements in the following outcomes:
The new guideline encourages holistic treatment and will impact current practice; an increase in numbers of counselling/consultation episodes (including time taken) may result. Professionals are expected to be qualified to provide high-quality spirometry and interpretation for appropriate diagnosis and earlier management. Prescribing will change, replacing single long-acting inhaled agents with dual (increasing LABA/LAMA prescribing) and reducing inappropriate ambulatory and short-burst oxygen prescribing. Smoking cessation intervention should increase.
Close communication will be required across sectors to ensure continued prescribing, monitoring and review of medications, especially for macrolides and oxygen. There is an expected increase in referrals for LVR interventions. Increased monitoring and pharmacovigilance will be necessary to minimise and manage medication adverse effects. Greater patient empowerment and increased self-management plans are expected (tailoring therapy for maximal benefit and reducing hospitalisation).
The NICE guideline has been long overdue; it conflicts with the most recent 2019 GOLD COPD guidance on prevention, diagnosis and management, which might cause clinicians some confusion as to which guideline to use. GOLD provides pragmatic guidance such as acknowledging the potential role of eosinophils to inform ICS prescribing and is used globally; hence all suggestions might not be applicable/available to UK patients.
NICE has a robust development process, leading to evidence-based recommendations for the most cost-effective interventions to provide maximal societal value benefit. It reiterates the importance of non-pharmacological measures to underpin the overall holistic treatment approach before escalating therapy. It acknowledges the need for appropriately skilled healthcare professionals to be able to diagnose, monitor and review patients with COPD throughout their disease trajectory. This should empower multidisciplinary staff (such as physiotherapists and pharmacists) to expand current roles, enabling patients’ greater access for review, support and consistent messages of disease management.
The new guidance takes a more considered approach to ICS use. Fixed dose triple inhalers are now available and the role of inhaled triple therapy is becoming clearer; further guidance on this is due in Summer 2019. The guidelines encourage more cost effective, responsible prescribing overall including improving medication adherence and a reduction in waste, which can be the basis for further quality improvement work.
The effect of long-term macrolide antibiotic use is unknown, both in terms of safety and clinical effect, there are no studies beyond one year to inform this. There is also concern of increased antimicrobial resistance as a result. It is expected that this intervention will have little cost implication but may reduce exacerbations and associated costs of health resource utilisation. Patients will need to be monitored closely with increased counselling, pharmacovigilance and ‘yellow card’ reporting to the Medicines Health Regulatory Authority (https://yellowcard.mhra.gov.uk/).
The guidance addresses many current clinically relevant issues in the diagnosis and management of patients with COPD but acknowledges that evidence is still lacking or unclear in some areas, leading to recommendations for research.
COPD has no cure but if guidance is applied, in conjunction with the quality standards, premature mortality can be prevented. Patients can have better healthcare experiences, co-create their care decisions for an improved quality of life and palliation. See the full guideline for detailed guidance and recommendations on the above.
References
1 Department of Health. COPD commissioning toolkit, a resource for commissioners. Department of Health, England, August 2012. www.gov.uk/government/uploads/system/uploads/attachment_data/file/212876… (accessed May 2019).
2 National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management NICE guideline [NG115] Published date: December 2018. www.nice.org.uk/guidance/ng115 (accessed May 2019).
3 Global Strategy for Prevention, Diagnosis and Management of COPD. 2019 report – Global initiative for chronic obstructive lung disease. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14N… (accessed May 2019).
4 Chen WC et al. Long-acting beta2-agonists versus long-acting muscarinic antagonists in patients with stable COPD: A systematic review and meta-analysis of randomized controlled trials. Respirology 2017;22:1313–19.
5 Vogelmeier C et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011;364:1093–103.
6 National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2019 update). In development [GID-NG10128]. www.nice.org.uk/guidance/gid-ng10128/documents/draft-guideline (accessed May 2019).
7 National Institute for Health and Care Excellence. Depression in adults: recognition and management Clinical guideline [CG90] Published date: October 2009. Last updated: April 2018. www.nice.org.uk/guidance/cg90 (accessed May 2019).
8 National Institute for Health and Care Excellence. Depression in adults with a chronic physical health problem: recognition and management. Clinical guideline [CG91] Published date: October 2009. www.nice.org.uk/guidance/cg91 (accessed May 2019).
9 Luckett T et al. Contributions of a hand-held fan to self-management of chronic breathlessness. Eur Resp J 2017;50:1700262; DOI: 10.1183/13993003.00262-2017
10 National Institute for Health and Care Excellence. Stop smoking interventions and services NICE guideline [NG92] Published date: March 2018. www.nice.org.uk/guidance/ng92 (accessed May 2019).
11 Bauld L et al. The effectiveness of NHS smoking cessation services: a systematic review. J Publ Health 2009:1–12.
12 Hoogendoorn M et al. Long-term effectiveness and cost effectiveness of smoking cessation interventions in patients with COPD. Thorax 2010;65:711–18.
13 Public Health England. Immunisation against infectious disease — ‘The Green Book’.
14 British National Formulary. Number 76. September 2018 – March 2019. British Medical Journal Group and Royal Pharmaceutical Society (Great Britain).
15 Barnes H et al. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Systematic Review – Intervention Version published: 31 March 2016. www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011008.pub2/full#CD0… (accessed May 2019).
16 National Institute for Health and Care Excellence. Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence Clinical guideline [CG76] Published date: January 2009. www.nice.org.uk/Guidance/CG76 (accessed May 2019).
17 National Institute for Health and Care Excellence. Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes NICE guideline [NG5] Published date: March 2015. www.nice.org.uk/guidance/ng5 (accessed May 2019).
18 National Institute for Health and Care Excellence. Multimorbidity: clinical assessment and management NICE guideline [NG56] Published date: September 2016. www.nice.org.uk/guidance/ng56 (accessed May 2019).
19 National Institute for Health and Care Excellence. End of life care for adults. Quality standard [QS13]. Published date: November 2011. Last updated: March 2017. www.nice.org.uk/guidance/qs13/chapter/List-of-statements (accessed May 2019).
20 National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in adults. Quality standard [QS10]. Published date: July 2011. Last updated: February 2016. www.nice.org.uk/guidance/qs10 (accessed May 2019).
Asthma is a common respiratory condition, affecting 1–18% of the population in different countries. It is characterised by symptoms such as breathlessness, wheeze, cough and chest tightness. Typically, these symptoms vary over time, reflecting the variable expiratory airflow limitation that underpins its diagnosis. The variations in symptoms and airflow limitation can be caused by temporary (or persistent) exposure to trigger factors such as allergens or irritants, exercise, change in weather, or viral respiratory infections.1
The long-term goals of asthma management include symptom control, normal activity levels, as well as reducing the risk of exacerbations. This is achieved using both pharmacological and non-pharmacological treatments, in a step-wise manner. Severe asthma is defined as asthma that requires Step 4 or 5 treatment, which includes medium- or high-dose inhaled corticosteroid (ICS) in conjunction with long-acting beta-agonist (LABA). Severe asthma may also require additional medications such as leukotriene receptor antagonists (LTRA), theophylline, anti-immunoglobulin E (anti-IgE), anti-interleukin 5 (anti-IL5), low dose oral corticosteroids (OCS) and tiotropium.1
Targeted lung denervation (TLD) is a novel bronchoscopic treatment that has been trialled in patients with moderate to severe chronic obstructive pulmonary disease (COPD), with early indications that it could be of benefit. In a randomised, double-blind, sham-controlled study, TLD demonstrated a significant reduction in respiratory adverse events and a tendency towards improved quality of life, dyspnoea and pulmonary function when compared with the sham arm.2 It uses a radiofrequency (RF) ablation catheter to disrupt the pulmonary connections of the vagus nerve with a minimally-invasive technique. As a result, acetylcholine release on airway smooth muscle and submucosal glands is curtailed, causing a reduction in the cholinergic effects of bronchoconstriction and mucous secretion respectively. All pulmonary parasympathetic innervation stems from the pulmonary trunks of the vagus nerves as they enter the lung at the hila.3 During TLD, nerve ablation occurs at the main bronchi and therefore it exerts its actions on airways downstream from this treatment site. At the time of ablation, the airway epithelium is prevented from the effects
of excessive heating by a coolant-filled balloon, which is situated adjacent to the ablation electrode.
The rational for TLD in COPD is the putative pathological increase in vagus nerve input to the smooth muscle cells and submucosal glands of the airways. It has been reported that enhanced parasympathetic activity is the dominant reversible component of airway obstruction in COPD.4 Treatment to address this component has already been developed and the current state of the art is tiotropium bromide, a muscarinic antagonist. While tiotropium improves airflow obstruction, only 50% of patients derive a clinically significant benefit.5 Furthermore, only 40% of patients persist with tiotropium after one year of starting treatment.6 Tiotropium does provide sustained bronchodilation over twenty-four hours, hence its use as one of the most common maintenance therapies in COPD. However, there are still significant changes in the FEV1 over the course of the day, with trough values being half those of peak values.7
Tiotropium, like all other inhalers, is affected by problems with drug deposition and duration of action. Tiotropium and TLD act by blocking the action of the vagus nerve on the airways, the former via a pharmacological mechanism, and the latter through a more direct physical approach. Pharmacological treatment is also dependent on patient compliance. The physical approach has the theoretical advantage of bronchodilating all airways distal to the main bronchus (including the smaller, peripheral airways), as well as having a constant effect over the course of the day and night. It has already been stated that tiotropium is a treatment option for asthma. Is there an argument, therefore, for TLD as a potential therapy in asthma?
Before we can answer this question, we must first understand the role of the vagus nerve in the pathophysiology of asthma. One way to elucidate its contribution is to assess the effects of temporarily blocking its actions. This was carried in a small study that measured the effect of intravenous atropine on nocturnal airflow limitation in ten asthma patients with known diurnal variation in peak expiratory flow (PEF). Atropine is a naturally-occurring compound and a competitive antagonist of muscarinic cholinergic receptors. Intravenous atropine administration would therefore cause systemic disruption of parasympathetic pathways, including those supplied by the pulmonary trunks of the vagus nerve. Both nocturnal and daytime PEF significantly improved after atropine. Whilst the nocturnal fall in PEF was not completely abolished, it was certainly diminished. The proposed mechanism was speculative, but it was suggested that the normal circadian effects of the vagus nerve are exaggerated by hypersensitisation of airway muscarinic receptors by inflammatory mediators.8 Therefore blocking the actions of the nerve (pharmacologically or physically) could help to limit nocturnal airflow obstruction, a phenomenon which causes symptoms in many asthma patients.
TLD is not the first procedure proposed for asthma that involves section of airway nerves.
In 1923, the German surgeon Professor Hermann Kümmell reported the first surgical intervention on the nerve supply to the lungs for asthma.9
He actually performed a unilateral cervical sympathectomy in the belief that some vagal fibres enter the lung via the sympathetic trunk.
It was subsequently postulated that the sympathetic trunk is the afferent part of a reflex arc, of which the efferent component is the bronchoconstriction-inducing parasympathetic vagal fibres.3 Kümmell’s report documented immediate relief of asthma after the surgery.
This led to the uptake of similar procedures in other centres. In 1929, Phillips and Scott published a review of cases of operations to the pulmonary nerves. They found over 300 cases had been performed since 1923, but only 29 had been reported in sufficient detail and had undergone at least 6 months follow-up. Of these, 8 (28%) were ‘cured,’ 5 (17%) were ‘improved,’ and 16 (55%) were ‘unimproved.’10 Most of these procedures involved intervention to the sympathetic trunk, with few vagotomy procedures.
Vagal section for asthma underwent something of a revival in the 1950s. Blades et al carried out procedures that involved destruction of the pulmonary plexus around the main bronchus, as well as division of the vagus nerve below the level of the recurrent laryngeal nerve. Of the 38 patients treated, 22 saw a resolution or improvement in their asthma, though 7 patients died.11 Dimitrov-Skokodi et al performed 19 cases of vagotomy and sympathectomy. They reported asthma attacks ceased altogether in ten patients and were reduced in seven. There were also improvements in mucosal oedema, sputum volume and eosinophilia, radiographic ‘emphysema,’ bronchographic bronchospasm and forced vital capacity.12 Rienhoff and Gay described bilateral pulmonary plexus resection in 11 patients. Results were very similar to Dimitrov-Skokodi’s results, with a reduction in the severity and frequency of asthma attacks, reduced sputum volume, and resolution of radiographic ‘emphysema.’13 Given the period these reports were published in, the assessment of outcomes are mostly subjective with little objective physiological data. It was also prior to the onset of randomised controlled trials, with the reports mostly published as case series and therefore prone to measurement bias. Nonetheless, it cannot be dismissed that a significant proportion of patients reported subjective improvement of their asthma, and that this was the experience across several different treating centres.
The literature on surgical intervention for asthma is more sparse after the 1950s, coinciding with improvements in pharmacological treatments during this period. Inhaled anti-vagal medications (that is, antimuscarinics) are introduced in asthma later in the century in the form of atropine14 and ipratropium bromide.15 The most established of the antimuscarinics in asthma is tiotropium bromide, which has also been used in COPD maintenance therapy for
a number of years. Tiotropium’s success has been attributed to its kinetic selectivity for the M1 and M3 muscarinic receptors. It dissociates from these receptors much slower (around 100-fold) than from the M2 receptor, a prejunctional autoinhibitory receptor that restricts acetylcholine (ACh) release when activated.
By allowing the M2 receptor to resume its action as the ‘handbrake’ of ACh-induced bronchoconstriction, whilst providing durable M3 receptor antagonism, tiotropium has an advantage over its non-selective antimuscarinic counterparts atropine and ipratropium bromide.16 Tiotropium has been shown to reduce exacerbations and improve lung function in asthma poorly controlled on an inhaled corticosteroid and long-acting beta-agonist. In a randomised placebo-controlled trial, tiotropium improved FEV1 by 154ml and reduced severe exacerbations by 21%.17 It has also been shown to improve symptoms and lung function in patients uncontrolled on inhaled corticosteroids alone.18
The reduction in exacerbations by both tiotropium and surgical denervation leads to the exciting possibility that anti-vagal therapies cause a reduction in airways inflammation. From animal models and in vitro studies, acetylcholine has been shown to have a role in allergen-induced airways inflammation and remodelling.19 Furthermore, a pilot study of TLD in COPD showed reductions in neutrophils as well as the chemokines CXCL8 and CCL4 at 30 days post-treatment. RNA profiling also highlighted reduced gene expression of TGF-β, IL-6 and MUC5AC.20
TLD is a treatment in its infancy. The evidence base for TLD is limited in COPD, and even more so in asthma. Here, we have attempted to make a case for a denervation procedure in asthma. Invasive interventions for obstructive airways diseases have not always, and perhaps still do not have the uptake that their respective evidence bases should afford them. Lung volume reduction in emphysema and bronchial thermoplasty in asthma are two examples of such procedures.
The anti-vagal therapies described above illustrate the benefits that this approach can potentially confer in severe asthma. In particular, while the surgical denervation data are not of the high scientific quality we expect in the current age of evidence-based medicine, it certainly does enough to stoke our interest and curiosity into the potential value of a denervation procedure of some form. A safety and feasibility trial of TLD
in severe asthma (NCT02872298) is currently recruiting across Europe and its results are eagerly awaited.
References
1 Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. www.ginasthma.org. 2018.
2 Slebos D-J et al. A double-blind, randomized, sham-controlled study of Targeted Lung Denervation in patients with moderate to severe COPD. Eur Respir J 2018;52(suppl 62):OA4929.
3 Kuntz A, Louis S. The autonomic nervous system in relation to the thoracic viscera. Chest 1944;10(1):1–18.
4 Gross N, Skorodin M. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med 1984;311(7):421–5.
5 Tashkin D et al. A 4-Year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med.2008;359(15):1543–54.
6 Breekveldt-Postma NS et al. Enhanced persistence with tiotropium compared with other respiratory drugs in COPD. Respir Med 2007;101(7):1398–1405.
7 Van Noord JA et al. Assessment of reversibility of airflow obstruction. Am J Respir Crit Care Med 1994;150(2):551–4.
8 Morrison JF, Pearson SB, Dean HG. Parasympathetic nervous system in nocturnal asthma. Br Med J (Clin Res Ed) 1988;296(6634):1427–9.
9 Kummell H. Die operative Heilung des Asthma Bronchiale. Klin Wochenschr. 1923;2(40):1825–7.
10 Phillips EW, Scott WJM. The surgical treatment of bronchial asthma. Arch Surg 1929;19(6):1425–56.
11 Blades B, Beattie E, Elias W. The surgical treatment of intractable asthma. J Thorac Surg 1950;20(4):584–91.
12 Dimitrov-Szokodi D, Husveti A, Balogh G. Lung denervation in the therapy of intractable bronchial asthma. J Thorac Surg 1957;33(2):166–84.
13 Rienhoff W, Gay L. Treatment of intractable bronchial asthma by bilateral resection of the posterior pulmonary plexus. Arch Surg 1938;37(3):456–69.
14 Snow R et al. Inhaled atropine in asthma. Ann Allergy 1979;42(5):286–9.
15 Ward M et al. Ipratropium bromide in acute asthma. Br Med J (Clin Res Ed). 1981;282(10):598–600.
16 Barnes PJ et al. Tiotropium bromide (Ba 679 BR), a novel long-acting muscarinic antagonist for the treatment of obstructive airways disease. Life Sci 1995;56(11-12):853–9.
17 Kerstjens HAM et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med 2012;367(13):1198–1207.
18 Peters SP et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med 2010;363(18):1715–26.
19 Kistemaker LEM, Gosens R. Acetylcholine beyond bronchoconstriction: Roles in inflammation and remodeling. Trends Pharmacol Sci 2015;36(3):164–71.
20 Kistemaker LEM et al. Anti-inflammatory effects of targeted lung denervation in patients with COPD. Eur Respir J 2015;46(5):1489–92.
The British Thoracic Society (BTS) macrolide guideline is the first of its kind dedicated solely to the use of oral macrolides, long term, in respiratory disease (where ‘long term’ refers to any duration longer than that used to treat an exacerbation). The dosing is ‘low dose’, that is, doses that are not used to ‘treat’ an acute exacerbation and are essentially prophylactic.
Macrolides are bacteriostatic antibiotics characterised by their large lactone ring structures with a broad spectrum of activity against many gram-positive bacteria. They were discovered in the 1950s when erythromycin was isolated from the soil bacterium Streptomyces erythraeus. In the 1970s and ‘80s, synthetic derivatives of erythromycin, including clarithromycin and azithromycin, were developed.
Macrolide antibiotics act by inhibiting protein synthesis of bacteria by binding to the 50S ribosomal element. Resistance occurs by several mechanisms. Clarithromycin and azithromycin are more active than erythromycin against several gram-negative bacteria as well as Mycoplasma pneumonia, Helicobacter pylori, Toxoplasma gondii, cryptosporidia and several atypical mycobacteria.1
Many studies were reviewed during the guideline development process and these used various different macrolide agents (predominantly clarithromycin and azithromycin). However, the greatest volume and best available evidence is related to the use of azithromycin, hence the recommendations made throughout the BTS guidance relate to azithromycin.
The guideline is applicable to adult patients and has some exclusions:
Disease areas covered by guideline are:
At the time of guideline development, no macrolide antibiotics are licensed in the UK for long-term, low-dose usage as immunomodulatory agents. Healthcare providers need to use clinical judgment, knowledge and expertise when deciding whether it is appropriate to apply recommendations for the management of patients. The recommendations cited are a guide and might not be appropriate for use in all situations. The guidance provided does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of each patient, in consultation with the patient and/or their guardian or carer.3
It is envisaged that initiation of macrolides will occur in a secondary or tertiary care setting.
The evidence reviewed for macrolide use in asthma presented some issues; for example, there was great heterogeneity of study designs, many were of a small size or underpowered. Studies ranged in duration from 6–12 months and some used exacerbations as a primary outcome and with varying definitions of what constituted an exacerbation; other studies used this as a secondary outcome measure. Hence there was some limit to applicability of evidence to the wider asthma population.
There were two large trials upon which the recommendations for azithromycin use were based: the AMAZES and AZIZAST trials (both studies also had slightly differing definitions of an exacerbation).
Overall there was a clear reduction in asthma exacerbations (both moderate and severe). This was seen for both eosinophilic and non-eosinophilic exacerbations with a greater reduction seen in eosinophilic groups.
The majority of studies demonstrated improvement in asthma symptoms. Actual changes were minimal and unlikely to be of clinical significance (statistical significance was reached in only three studies).
Azithromycin use may reduce bronchial hyper-responsiveness in asthma and might result in a reduction in oral steroid dose, but this is not a consistent finding. It can result in a small improvement in lung function and peak expiratory flow rate; it may also result in measurable improvements in quality of life (QoL) but the clinical impact of these changes remains unknown and may be very small.
There is no evidence of the impact of macrolide therapy on mortality, exercise capacity, disease progression or sputum production in people with asthma, therefore no recommendations in regard to these outcomes can be made in this guideline.
Recommendation
Oral macrolide therapy should be considered to reduce exacerbation frequency in adults (50–70 years), with ongoing symptoms despite >80% adherence to high-dose inhaled steroids (>800mcg/day of beclomethasone diproprionate equivalent) and at least one exacerbation requiring oral steroids in the past year. This recommendation reflects the population within the AMAZES4 randomised, controlled trial (RCT), which represents the highest quality evidence of macrolide therapy leading to a significant reduction in exacerbations. Treat for 6–12 months’ duration, 500mg three-times a week.
There were nine RCTs reviewed, ranging from 3 to 12 months’ duration and using different macrolides. The mean ages of participants were 64–72 years. There was no evidence of improvement in lung function and no mortality benefit. There was a statistically significant improvement in QoL measured by the St George’s Respiratory Questionnaire (SGRQ), but not clinically significant four-unit improvement as minimum clinically important difference. The number of hospitalisations was not significantly reduced in patients receiving long term macrolide therapy.
Recommendations
Long-term macrolide therapy should be offered to patients with COPD who are non-smokers, with three or more acute exacerbations requiring steroid therapy and/or one exacerbation requiring hospital admission per year to reduce exacerbation rate (strong)
Note – this differs slightly to the recommendations in the recent NICE COPD 2018 guidance,6 which states:
‘Consider azithromycin (usually 250mg three-times a week) for people with COPD if they:
There were three main RCTs that were the basis of recommendations (BAT, BLESS and EMBRACE). They all studied use of a macrolide versus placebo in bronchiectasis. Studies ranged from 6 to 12 months’ duration and had different entry criteria. Mean ages of participants were 60–62 years.
The studies showed a reduction in the number of exacerbations and some symptom improvement.
Long-term macrolide therapy may reduce sputum volume and weight. There was some sputum reduction but the impact of this to patients was unclear and there was no impact on exercise capacity.
There was little change in QoL but there is evidence of an improvement in QoL measured by the SGRQ when azithromycin 250mg daily is used for one year.
Dosing regimens with greatest supportive evidence for exacerbation reduction are azithromycin 250mg OD, azithromycin 500mg three-times a week and erythromycin ethylsuccinate 400mg BD. Studies with other dosing regimens, including azithromycin 250mg three-times a week (as pragmatically suggested in the BTS bronchiectasis guideline) have also reported reduction in exacerbations but have a lower evidence base.
Studies with greatest evidence for reduction in exacerbations used therapy for a minimum of six months; the impact beyond one year is unknown. There is evidence for reduction in exacerbations over a 12-month period when therapy is used for six months and then not for the subsequent six months but the impact of subsequently recommencing is unknown.
Recommendations
Long-term macrolide treatment should be considered to reduce exacerbations in those with high exacerbation rates (that is, three or more per year). Therapy should be for a minimum of six months. The impact beyond 12 months is unknown. The dosing regimens with the greatest supportive evidence, when using macrolides to reduce exacerbation rates, are azithromycin 250mg daily, azithromycin 500mg three-times a week and erythromycin ethylsuccinate 400mg twice a day. However, a starting dose of 250mg three-times a week can be used to minimise side effect risk with subsequent titration according to clinical response.
The overall quality of the evidence is at best modest in this area. Bronchiolitis obliterans syndrome (BOS) is a devastating complication of lung transplantation, hence any intervention offering the chance of prevention, reversal or stabilisation is welcome. Long-term macrolide use is a low-risk intervention. On this basis, two low evidence recommendations have been made.
Recommendations
Recommendations against macrolide use
Potential adverse effects requiring monitoring:7
(Note: This is not an exhaustive list – consult the BNF or Summary of Product Characteristics for more in-depth information on adverse effects).
The BTS macrolide guidelines should be used in conjunction with the relevant disease guideline (for example, with BTS bronchiectasis guide). Holistic care (both pharmacological and non-pharmacological) should be optimised first, ensuring that patients are being treated appropriate to disease stage/severity and that individual circumstances have been taken into account. This includes comorbidities, polypharmacy, addressing non-adherence, and ensuring smoking cessation before considering macrolides.
Prior to macrolide initiation, in-depth patient consultation and counselling are required to present treatment risks and benefits, discuss side effects, review potential or actual interactions (especially during exacerbation) and to manage expectations of therapy.
Azithromycin is principally cleared by the liver so should be used with caution in significant liver disease. It can be taken with or without food at any time of day but indigestion remedies should be avoided for two hours before the azithromycin dose and if taking as a coated tablet, must be swallowed whole not chewed or crushed.
Electrolyte disturbances and medicines such as amiodarone, fluoroquinolones and some antidepressants/antipsychotics may potentiate QT prolongation.7
The guideline will be published with an associated generic patient information leaflet that can be locally adapted and which will include the basic information to cover during counselling.
Close communication will be necessary across primary, secondary/tertiary care to ensure clear shared care and follow up plans are developed and followed, ensuring that prescribing and monitoring are ongoing. Close liaison with the healthcare team (in particular with pharmacists) is also necessary to ensure recognition of adverse effects and manifestation of any medicine interactions (recognition more so during an exacerbation where acute therapies may interact). This is especially important during the initial phase after starting therapy when patients may present to primary care providers or acute services.
Increased discussion and rapport with patients will support the increased pharmacovigilance required and also for potential Medicines and Healthcare products Regulatory Authority ‘Yellow card’ reporting. Antimicrobial stewardship is also necessary.
The use of macrolides during disease exacerbation is unclear and evidence for this is lacking. It may be appropriate to hold the macrolide during exacerbation, for example, in bronchiectasis where the patient may require intravenous antibiotics. Some experts believe that patients should not be treated with full dose macrolide during an exacerbation if on prophylaxis, however some clinicians acutely increase the dose of the same agent. Current practice varies widely across the UK.
Limitations of the available macrolide evidence mean we do not have any data on long term effect, not only in terms of safety and clinical effect but also antimicrobial resistance and the microbiome. There is no long-term data on use beyond one year or head-to-head comparison between the different macrolides to show superiority of one over another.
The guidelines raise relevant research questions such as: which disease phenotypes/subgroups would gain most benefit from macrolides? Is seasonal use of macrolides or rotation with other antibiotics beneficial? What is the potential for use in a younger population (study populations were older)? Also, is the threshold for use that has been suggested appropriate? Would this need to be tightened or relaxed? Further research will inform these questions.
The new BTS macrolide guidelines will provide much needed guidance for the use of macrolides as immunomodulatory agents across various respiratory disease areas. However, guidelines are a guide and common sense applies – practitioners should ‘use clinical judgment, knowledge and expertise’ before initiation, taking into account individual patient needs and choice. Macrolides appear to reduce inflammation, bronchial hyperreactivity and exacerbations and may affect other parameters too. They are not a magic bullet – all other treatments should be optimised first and they should be avoided in current smokers (ineffective).
References
1 National Institutes of Health. Drug Record. Macrolide antibiotics. https://livertox.nih.gov/MacrolideAntibiotics.htm (accessed August 2019).
2 National Institute for Health and Care Excellence. Cystic fibrosis: long-term azithromycin. Evidence summary ESOUM37. www.nice.org.uk/advice/esuom37/chapter/Key-points-from-the-evidence (accessed August 2019).
3 British Thoracic Society. Long-term macrolide guideline 2019. www.brit-thoracic.org.uk/quality-improvement/guidelines/long-term-macrol… (accessed August 2019).
4 Gibson PG et al. Effects of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES) a randomised, double blind, placebo- controlled trial. Lancet. 2017;390(10095):659–68.
5 Albert RK et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011;365:689–98.
6 National Institute for Health and Care Excellence. NG115. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. December 2018 www.nice.org.uk/guidance/ng115 (accessed August 2019).
7 British National Formulary https://bnf.nice.org.uk/ (accessed August 2019).
10th September 2019
In England in 2013, there were 28,000 cases where emergency medical services (EMS) were called to attempt resuscitation for out of hospital cardiac arrest (OHCA).1 Other large studies give an incidence of OHCA of approximately 80 per 100,000 patient years.2 Worldwide, this figure is significantly higher than in Asia but falls slightly behind the USA and Australia.2 These numbers are only the amount of cases where EMS were called to OHCA, not how many were treated, or indeed how many had a cardiac cause, or
how many of those with cardiac cause were found to be in ventricular fibrillation (VF). This is important as there are positive survival rates associated with VF cases of OHCA versus any other.3
Mild therapeutic hypothermia (MTH), or targeted temperature management (TTM) as it is now more commonly known, has been established for the post-resuscitation care of OHCA since two pivotal studies in 2002.4,5 In the UK, it is incorporated into Resuscitation Council guidelines as the fourth link in the chain of survival (post-resuscitation care; Figure 1). This was supported by the International Liaison Committee on Resuscitation (ILCOR) in 2015.6
This review aims to look at the theory behind TTM and how it is applied clinically, with attention to new research in this field and what impact TTM has on patient survival and neurological recovery.
Hippocrates and the Persian army had already commented on the protective nature of cold for centuries before Napoleon’s chief surgeon, Dominique Jean Larrey, documented that injured soldiers kept closer to the fire died more frequently than those kept away.7 Studies from the middle of the 20th century looked into MTH following both severe head injury and cardiac arrest,8,9 but the results were uncertain and not incorporated into standard practice.
Pivotal case reports exist of survival from acute hypothermia with intact neurology, possibly none more dramatic than that of Anna Bagenholm, trapped under the ice in Norway for 80 minutes after a skiing accident in 1999, and surviving to this day to work as a doctor. Her core temperature was recorded at 13.7°C on arrival at hospital, and her heart started after slow rewarming on cardiac bypass. It is only in the last 15–20 years that studies have progressed to looking not only at survival rates post-OHCA but also what degree of neurological deficit exists after TTM is instigated.
Understanding that the damage to the brain occurs after cardiac arrest from hypoxia is paramount. There is no substitute for early recognition, early CPR and early defibrillation as laid out by the Resuscitation Council to reduce morbidity and mortality for OHCA. However once primary neurological insult has occurred, there should be every effort made to reduce the secondary reperfusion injury to the brain.
The mechanism of this injury is thought to be governed by free radical formation and inflammatory mediators such as TNFα and interleukin-1.10 During this ischaemia–reperfusion time, it is suspected that ATP molecules are reduced in the brain and anaerobic glycolysis occurs, causing intracellular levels of phosphate, hydrogen and lactate to rise; this, in turn, leads to intra- and extracellular acidosis and subsequently cell apoptosis and death.10
It is known in animals that there is reduction in cerebral metabolic rate for oxygen (CMRO2) of 6% for every 1-degree reduction in brain temperature (>28°).11 It is theorised that the injured brain could be therefore be protected in this critical post-resuscitation period. There is also evidence that a 4–5-fold increase in oxygen radicals may be present in hyperthermia.12 Subsequently this is suggested to be a factor in why hyperthermic patients suffering OHCA have an increased mortality rate and incidence of neurological sequlae.13
While inducing hypothermia for TTM seems to convey protection, it is not without risk. The documented side effects for this treatment range from diuresis causing electrolyte disturbance, shivering (causing need for muscle relaxation, thus masking seizure activity) to cardiac dysrhythmia, bleeding and immune suppression. A review listed cardiovascular complications as the most likely to occur, but deemed this due to pre-existing ischaemic heart disease (IHD) exacerbated by the haemodynamic imbalance caused by TTM.14
One key area of complication with TTM is glucose homeostasis, specifically with insulin resistance and reduced insulin secretion leading to hyperglycaemia; this causes an increase in the rate of infections, neuropathy and renal failure. Overall morbidity and mortality has been shown to be higher with hyperglycaemia in the critically ill population and therefore close monitoring and appropriate management of hyperglycaemia should be undertaken with TTM.14 In our hospital, we use a concentrated dextrose infusion with variable rate insulin regime to control blood glucose levels within a targeted range.
The reason TTM has replaced MTH or TH is to place the importance on defining a temperature profile for a patient, manipulating their physiological response.15 It can be divided into three phases:
Research exists on the optimal time to instigate TTM, its length of duration and rewarming time, with some studies even performing pre-hospital MTH, unfortunately without benefit to long-term survival or disability.16
All early studies seemed to conclude that TTM should be started as early as is feasibly possible; however, a randomised, controlled trial did not prove any benefit in terms of speed to achieve MTH in comparison to TTM and avoidance of hyperthermia.17
There are currently three main techniques available for cooling post-OHCA. These are summarised below, and the availability of each varies depending on location and on financial circumstances:
Conventional cooling methods
These methods include crushed ice, ice bags and the infusion of cold fluid. These have the advantage of being cheap, mostly accessible, and can be used in combination with other methods. These methods however are somewhat crude, and while an evidence base for adequately lowering temperature is available,18 they can reduce temperature to undesired levels and be difficult
to use in maintenance of desired temperature range.
Surface cooling methods
These devices, probably the most commonly available, work by circulating cold fluid or air through either blankets or pads wrapped or applied to the patient. A number of devices exist: blankets such as the Curewrap with CritCool by MTRE, and Kool-Kit with Blanketrol III by Cincinnati Sub-Zero are popular. The InnerCool STX by Philips and Arctic Sun by Medivance are examples of pad devices, the latter currently being used in our hospital.
The advantages of these devices are that they are easy to apply and rapidly initiate treatment, while also offering auto-feedback mechanisms to adjust water or air temperature accordingly in keeping with patient assessment from skin and core temperature sensors.
Unfortunately shivering is most common with these devices,19 which might necessitate muscle relaxation. There is also the possibility of skin irritation and burns, although these are rare.19
Intravascular cooling methods
These methods involve the cannulation of a central vein which comes with its own risks and complications, namely infection, bleeding and thrombosis. At time of writing, the Thermoguard XP (Zoll) and InnerCool RTx (Philips) are the major devices in use. The Thermoguard has the added benefit of having a triple lumen infusion catheter combined with its cooling system avoiding the need for multiple lines. The greatest benefit ascertained with these devices is precise temperature control in maintenance and rewarming phases and less shivering.20
Conventional methods aside, a recent retrospective study looked at surface cooling methods versus intravascular cooling devices.21 The analysis identified there was no change in outcome in either mortality or neurological deficit between these two methods. Statistically there were significantly more patients out of temperature range while maintaining therapy in the surface cooling group, and an increase in median time spent outside targeted temperature as well. This work further supported earlier studies22 that advocated the use of either surface pads or intravascular devices for the induction of hypothermia but strongly recommended intravascular devices for maintaining target temperature.
The National Institute for Health and Care Excellence updated its advice on TTM in July 2017 to advocate the use of Arctic Sun over conventional or intravascular cooling methods, due to a combination of reduced risks associated and the potential for improved outcomes.
TTM is an extensively researched, effective, neuroprotective strategy with well established guidelines; however, confusion exists about the optimal duration and target temperature. At the time of writing, the most up-to date research would advocate starting TTM as soon as feasibly possible, but not setting low temperatures (that is, 33°C) and simply avoiding temperatures above 36°C.17 This obviously remains at the clinician’s discretion and temperatures between 35 and 36°C are commonplace.
While new devices and research into this treatment are evolving and being undertaken, it is important to reflect on the fundamentals. First, patient selection remains an often-overlooked area, and the authors urge vigilance in selecting those patients who are suitable for instigation of TTM and screening carefully for those likely to benefit from this therapy. To this point, if there is doubt regarding the initial rhythm of arrest, then TTM should be instigated and not withheld.
The clinician involved in the decision to commence TTM must pay attention to the precise control of temperature in all phases, and critically in the rewarming phase where a passive, non-controlled rise could have serious effects on outcome.
Finally, as with most evolving medical research, there is more work to be done, particularly now that 36°C temperatures are associated with equal outcomes to 33°C. Are we ready to accept normothermia and adopt cooling measures when we see a trend to hyperthermia?
References
1 London Ambulance Service Cardiac Arrest Annual Report 2012/2013. www.londonambulance.nhs.uk.
2 Berdowski J et al. Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation 2010;81(11):1479–87.
3 Perkins GD, Brace-McDonnell SJ. The UK Out of Hospital Cardiac Arrest Outcome (OHCAO) project. BMJ Open 2015;5(10):e008736.
4 Bernard SA et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346(8):557–63.
5 The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346(8):549–56.
6 Monsieurs KG et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 1. Executive summary. Resuscitation 2015;95:1–80.
7 Gajić V. [Forgotten great men of medicine – Baron Dominique Jean Larrey (1766-1842)]. Med Pregl 2011;64(1–2):97–100.
8 Benson DW et al. The use of hypothermia after cardiac arrest. Anesth Analg 1959;38:423–8.
9 Williams GR, Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg 1958;148(3):462–8.
10 Knot J, Moťovská Z. Therapeutic hypothermia after cardiac arrest – Part 1: Mechanism of action, techniques of cooling, and adverse events. Cor et Vasa 2012;54(4):e237–42.
11 Steen PA et al. Hypothermia and barbiturates: individual and combined effects on canine cerebral oxygen consumption. Anesthesiology 1983;58(6):527–32.
12 Globus MY-T et al. Detection of free radical activity during transient global ischemia and recirculation: Effects of intraischemic brain temperature modulation. J Neurochem 1995;65(3):1250–6.
13 Zeiner A et al. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch Int Med 2001;161(16):2007–12.
14 Soleimanpour H et al. Main complications of mild induced hypothermia after cardiac arrest: A review article. J Cardiovasc Thorac Res. 2014;6(1):1–8.
15 Vaity C, Al-Subaie N, Cecconi M. Cooling techniques for targeted temperature management post-cardiac arrest. Crit Care [Internet]. 2015 [cited 2017 Nov 21];19(1). www.ncbi.nlm.nih.gov/pmc/articles/PMC4361155/ (accessed July 2018).
16 Diao M et al. Prehospital therapeutic hypothermia after cardiac arrest: A systematic review and meta-analysis of randomized controlled trials. Resuscitation 2013;84(8):1021–8.
17 Nielsen N et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med 2013;369(23):2197–206.
18 Larsson I-M, Wallin E, Rubertsson S. Cold saline infusion and ice packs alone are effective in inducing and maintaining therapeutic hypothermia after cardiac arrest. Resuscitation 2010;81(1):15–19.
19 Mayer SA et al. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med 2004;32(12):2508–15.
20 Lyden PD et al. Intravascular cooling in the treatment of stroke (ICTuS): early clinical experience. J Stroke Cerebrovasc Dis 2005;14(3):107–14.
21 Glover GW et al. Intravascular versus surface cooling for targeted temperature management after out-of-hospital cardiac arrest – an analysis of the TTM trial data. Crit Care 2016;20(1):381.
22 Hoedemaekers CW et al. Comparison of cooling methods to induce and maintain normo- and hypothermia in intensive care unit patients: a prospective intervention study. Crit Care 2007;11(4):R91.
Chronic obstructive pulmonary disease (COPD) is a disease characterised by chronic airflow limitation, and progressive and persistent respiratory symptoms.1 Today, COPD is the third largest cause of mortality worldwide, and its incidence increases gradually with smoking status.2 Emphysema is one of the subtypes of COPD, and its treatment with standard drugs is often difficult because of alveolar tissue damage, loss of elastic recoil, air trapping and hyperinflation.3 Pulmonary function tests in emphysema patients show increases in total lung capacity (TLC) and residual volume (RV) in addition to the classical COPD findings. Standard treatment regimens for COPD normally include smoking cessation, inhaler therapy, breathing exercises, nutritional support, as well as oxygen therapy and non-invasive ventilation in patients with severe emphysema.1 Unfortunately, these treatments do not always yield satisfactory results in patients with severe emphysema. In such patients, hyperinflation, local air trapping and intrathoracic compression are notable problems that result in increased breathing difficulty and place an additional burden on the respiratory muscles.
There are, however, other treatment options for patients with severe emphysema, such as lung volume reduction surgery, bullectomy and lung transplantation.4 Surgery is only suitable for selected patients due to intraoperative risks, postoperative complications, and increased mortality.5 In the last decade, endoscopic methods providing similar results to those of lung volume reduction surgery have been investigated, and minimally invasive methods have been developed.
The new and alternative treatment modalities, which are known as endoscopic lung volume reduction (ELVR) procedures, exert their effects through five main mechanisms: reduction of airway trapping; reduction of bronchoconstriction; improvement of elastic recoil; induction of local inflammatory reactions; and airway blockage.6 These ELVR procedures are endobronchial valve (EBV), coil, thermal vapour ablation, bio-lung volume reduction, targeted lung denervation, and airway bypass stent. An expert panel report on the EBV and coil methods has been published,7 and the other three procedures are currently in development. With increasing published evidence of effectiveness and safety, ELVR techniques have been available in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines since 2017 and are recommended in a range of situations, from the presence of refractory conditions to maximal medical treatment regimens and pulmonary rehabilitation programs.1
The major indication for treatment with ELVR methods is COPD of GOLD stage III or IV with severe emphysema. Values of patient forced expiratory volume in 1 second (FEV1) should be between 15% and 50% (15–45% for coil), and findings indicating the presence of hyperinflation (TLC >100%, RV >175%, RV/TLC ≥0.58) are required. Although there is no clear evidence, it is recommended by experts that these therapies should be avoided in patients with hypercapnia (PCO2 >55–60mmHg) and pulmonary hypertension (PAB > 50mmHg).7 Patient results for the 6-minute walk test (6MWT) should be in the range of 150–450m. Furthermore, these expensive treatments are not recommended for active smokers. To be considered for ELVR treatment, patients should have quit smoking at least two months prior; cessation of smoking six months before is most ideal (Table 1).
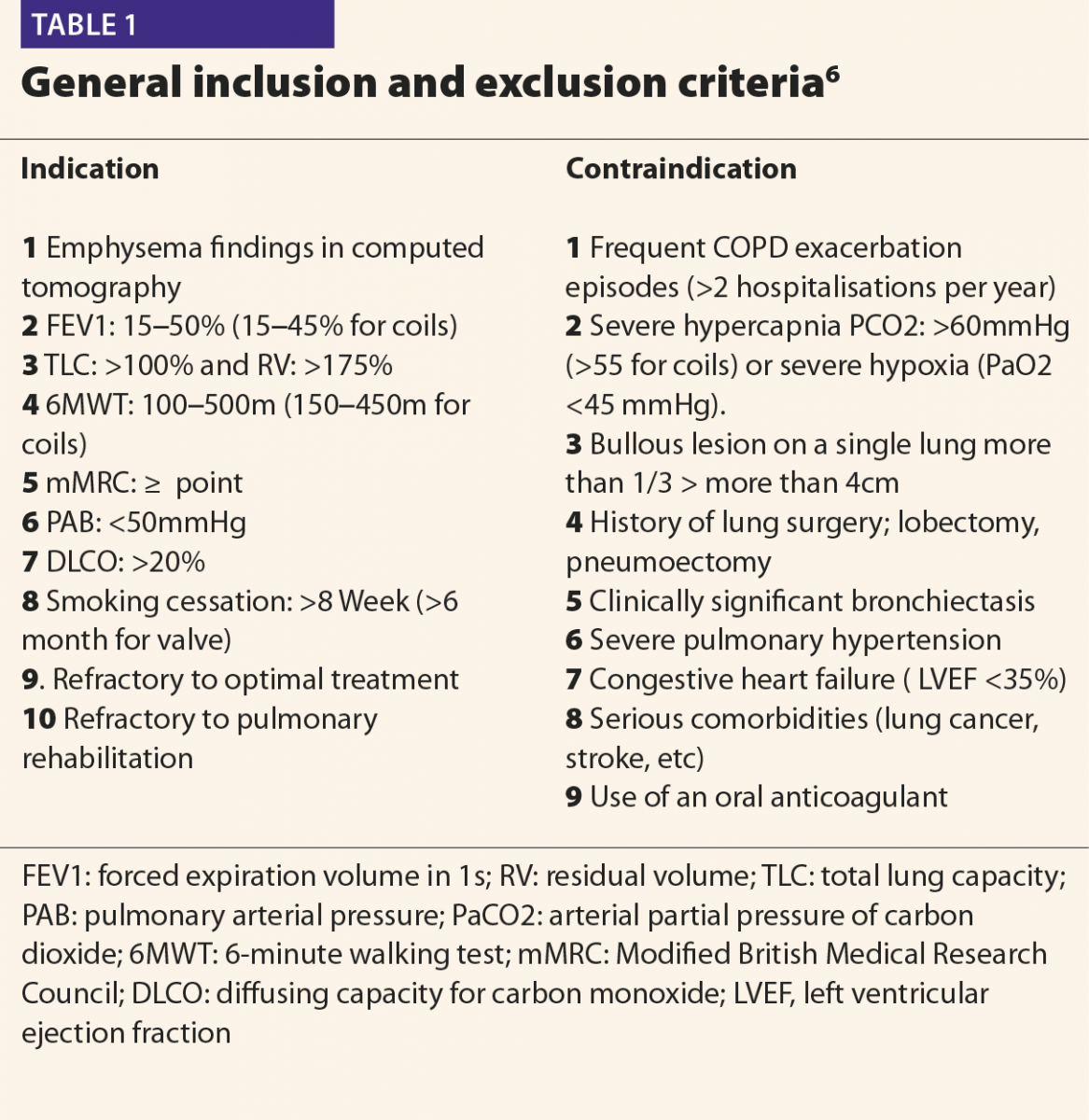
These treatments are not recommended for patients with clinically relevant bronchiectasis, lung cancer, bullous lesions greater than 4cm, history of prior lung surgery, severe hypoxia (PaO2 <45mmHg), hypercapnia (PCO2 >55–60), or in patients undergoing anticoagulation therapy.7 In addition, these treatments are not recommended in patients with impaired general status or in those who have comorbidities that might significantly affect survival. Other contraindications include unstable cardiovascular disease such as unstable arrhythmia, severe heart failure (left ventricular ejection fraction <35%), and stroke or myocardial infarction within the last six months.8 Although genetic α-1 antitrypsin deficiency has been accepted as a criterion for exclusion, discussion continues as to whether it should be considered as a contraindication.
The most frequently used and most evidence-based ELVR methods are the valve and coil procedures. Although their mechanisms of action are different, these methods can be performed in patients with both heterogeneous and homogenous emphysema. The treatment algorithm for these methods is shown in Figure 1.1,9 The valves are fully reversible, and coils are partially reversible, but it is not possible to recycle all applied coils.10
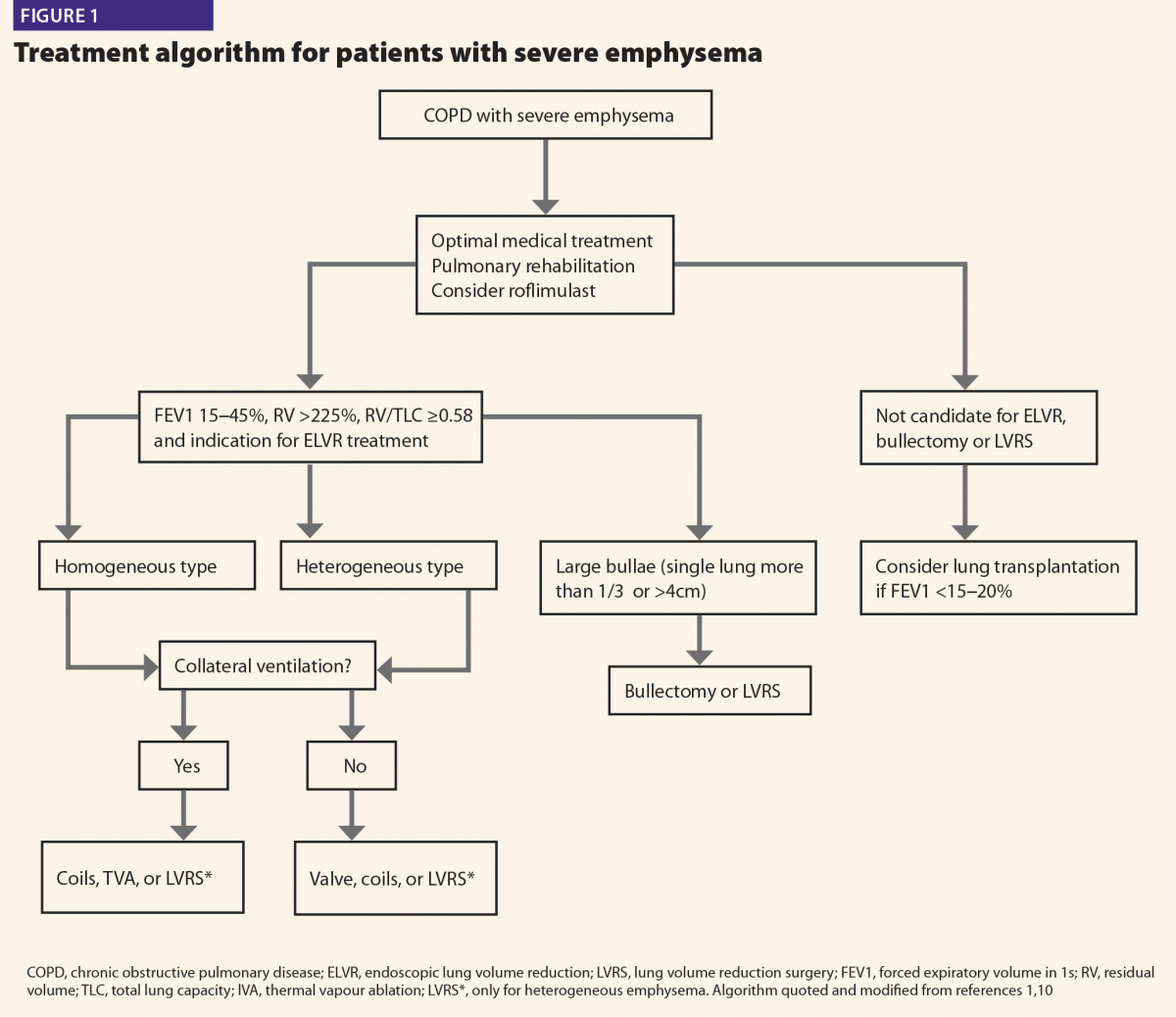
Endobronchial valve implantation
The evaluation of collateral ventilation and the fissure completeness score (FCS) is very important prior to treatment. These evaluations are performed by qualitative computed tomography and via the Chartis system during bronchoscopy. If the FCS and collateral flow are above 95%, valve therapy can be applied directly. Measurement with the Chartis system is required when collateral flow is between 80% and 95%. However, alternative endoscopic methods and surgeries should be considered in patients with collateral flow scores below 80%.7 Under light sedation, endobronchial valves are placed into bronchoscopically targeted segmental bronchi. Atelectasis and volume-reducing effects are achieved after blocking the hyperinflated lung lobe. Targeted lobar volume reduction is the efficacy outcomes measure, which is considered to be positive when a reduction of 350ml is achieved.11
Endobronchial coil implantation
The coil procedure (PneumRx Inc, Mountain View, CA, USA) was developed for patients with heterogeneous and homogenous emphysema with interlobar collateral ventilation. The procedure is performed under general anaesthesia and fluoroscopy. Approximately 8 and 14 nitinol coils (average of 10) are placed in the targeted lobe using bronchoscopy. The length of the airway is measured by the guide wire during the procedure, and 100, 125, and 150mm coils are used in the procedure. Using the carrier catheter, the coil is advanced to the targeted bronchus, but it is recommended not to place it too close to the central vascular structures and pleura.12
By pulling the catheter, the coil returns to its original shape before it is loaded into the catheter, thus reducing lung volume. The same procedure is applied in the other lung within four to eight weeks. Following the procedure, patients are kept under observation for one to two days.
In order to obtain a good response, the patient must have healthy parenchymal tissue in the non-targeted lung areas.6–8
In the studies conducted to date, EBV treatment resulted in an average increase in FEV1 values of 77.5ml (34.5–140.0ml), an increase in results of the 6MWT of 40.8m (9.3–91.0m), and an average decrease in RV values of 440ml (200–680ml).6 In addition, the following were observed: increased patient quality of life, a St George’s Respiratory Questionnaire (SGRQ) score decrease of 7.0 points, a modified Medical Research Council (mMRC) score decrease of 0.8 points, a BODE Index decrease of 1.2 points, and increased exercise tolerance.7
In a review of the coil procedure, it was reported that coil treatment resulted in an average increase in FEV1 values of 130ml (90–200ml), an increase in results for the 6MWT of 47.0m (14.6–84.0m), and an average decrease in RV values of 420ml (310–510ml).6 A significant increase in patient quality of life was also observed (SGRQ –12 points).7 Additionally, improvements in scores of anxiety and depression were recorded in this patient group.13 In a recent study, coil treatments were reported to cause improvements in blood gas parameters in patients with hypoxic or mild hypercapnic respiratory failure.14
Although ELVR therapies are minimally invasive procedures, they can lead to various complications due to the presence of COPD with severe emphysema. Pneumothorax (17.3%), valve migration (2.1%), pneumonia (1.7%), haemoptysis (1.9%), respiratory failure (1.4%), and exacerbations of COPD (0.9%) were reported in a recent study after application of EBV procedures.15 Pneumothoraces (86%) usually occur within the first 72 hours, indicating that patients should be hospitalised immediately for a minimum of 72 hours following the procedure.16
COPD exacerbation (41.0%) is the most frequent complication of coil procedures, followed by pneumonia (14.8%), and pneumothorax (5.7%).6 These complications may occur either immediately after the procedure or during follow-up. However, prophylactic antibiotics are frequently used by the bronchoscopist due to the high risk of COPD exacerbation and pneumonia. There is no general consensus on the best application of prophylactic antibiotics. Short-term steroid administration and stress ulcer prophylaxis are generally recommended to reduce the post-treatment acute inflammatory response.17
The EBV procedure is generally performed with mild sedation, and the coil procedure is performed with general anaesthesia in the operating room. Although patients are generally able to undergo the procedure, it is sometimes necessary to postpone it because a patient might not be able to tolerate the stress of anaesthesia.10
ELVR treatment methods are expensive. Cost estimates following an analysis were: valve method ($12,943); coil method ($11,328); and volume reduction surgery ($2444).18 These calculations do not include the costs associated with the management of complications and hospitalisation. Depending on agreements with health care systems, the cost of the Chartis catheter and delivery system for a single procedure can vary from $9185 to $10,300. However, total costs for one year of coil treatments were approximately $53,521 and $5912 for follow-up patients.19 In a landmark study (REVOLENS), the initial cost of coil therapy seemed very high, therefore, large-scale studies are required to better determine this method’s long-term cost and efficacy.19
Increasing ELVR treatment modalities have expanded the treatment spectrum for COPD patients with severe emphysema. In a group of patients with severe COPD, who were potential lung transplant candidates, positive results were obtained with ELVR treatment, indicating that ELVR treatment can act as a bridge until the time of transplantation.20 The most important reason for increasing treatment efficacy is correct patient selection. Although the EBV and coil treatments are the most commonly used methods, there is emerging positive evidence for other methods, such as thermal vapour ablation, bio-lung volume reduction, and targeted lung denervation. Autologous blood application will be performed much more frequently in the near future due to its low cost and easy applicability.21 Most recently, a newly designed device known as the ‘reverser’, which is similar to the coil structure, was tested in pigs, and positive results were obtained.22 The creation of treatment algorithms, research on the long-term effects of these methods, prevention and control of complications, and appropriate development of these methods are the most important issues ahead.
References
1 Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2017. www.goldcopd.org/ (accessed July 2019).
2 Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med 2013;369:448–57.
3 Holloway RA, Donnelly LE. Immunopathogenesis of chronic obstructive pulmonary disease. Curr Opin Pulm Med 2013;19:95–102
4 Marchetti N, Criner GJ. Surgical approaches to treating emphysema: Lung volume reduction surgery, bullectomy, and lung transplantation. Semin Respir Crit Care Med 2015;36: 592–608.
5 Criner GJ et al. The National Emphysema Treatment Trial (NETT) Part II: Lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med 2011;184:881–93.
6 Gülşen A. Bronchoscopic lung volume reduction: A 2018 review and update. Turk Thorac J 2018;19:141–9.
7 Herth FJF et al. Endoscopic lung volume reduction: An expert panel recommendation – Update 2019. Respiration 2019;97(6):548–57.
8 Slebos DJ et al. Endobronchial valves for endoscopic lung volume reduction: Best practice recommendations from Expert Panel on Endoscopic Lung Volume Reduction. Respiration 2017;93:138–50.
9 Gordon M, Duffy S, Criner GJ. Lung volume reduction surgery or bronchoscopic lung volume reduction: is there an algorithm for allocation? J Thorac Dis 2018;10:2816–23.
10 Gulsen A et al. Evaluation of bronchoscopic lung volume reduction coil treatment results in patients with severe emphysema. Clin Respir J 2017;11:585–92.
11 Welling JBA et al. Minimal important difference of target lobar volume reduction after endobronchial valve treatment for emphysema. Respirology 2018;23:306–10.
12 Slebos DJ et al. Endobronchial coils for endoscopic lung volume reduction: Best practice recommendations from an Expert Panel. Respiration 2018;96:1–11.
13 Bostancı K et al. Endobronchial coils in treatment of advanced emphysema: A single center experience. Turk Gogus Kalp Dama 2019;27:57–62.
14 Gülsen A. Effects of bronchoscopic lung volume reduction coil treatment on arterial blood gases. J Bronchology Interv Pulmonol 2019;26(2):90–5.
15 Fiorelli A et al. Complications related to endoscopic lung volume reduction for emphysema with endobronchial valves: results of a multicenter study. J Thorac Dis 2018;10:3315–25.
16 Gompelmann D et al. Clinical and radiological outcome following pneumothorax after endoscopic lung volume reduction with valves. Int J Chron Obstruct Pulmon Dis 2016;11:3093–9.
17 Herth FJ et al. Assessment of a novel lung sealant for performing endoscopic volume reduction therapy in patients with advanced emphysema. Expert Rev Med Devices 2011;8:307–12.
18 Metin B et al. A single-center comparison of the cost analyses of different volume reduction-therapy methods in the treatment of emphysema. Cukurova Med J 2017;42:721–79.
19 Deslée G et al; REVOLENS Study Group. Lung volume reduction coil treatment vs usual care in patients with severe emphysema: The REVOLENS Randomized Clinical Trial. JAMA 2016;315:175–84.
20 Gulsen A. Importance of bronchoscopic lung volume reduction coil therapy in potential candidates for lung transplantation. Biosci Trends 2018;12:395–402.
21 Bakeer M et al. Low cost biological lung volume reduction therapy for advanced emphysema. Int J Chron Obstruct Pulmon Dis 2016;11:1793–800.
22 Hu Y et al. A new-designed lung-bending device for bronchoscopic lung volume reduction of severe emphysema: A feasibility study in pigs. Respiration 2019;97(5):444–50
9th September 2019