This website is intended for healthcare professionals only.
Take a look at a selection of our recent media coverage:
12th September 2019
Delivery of high-quality CPR is the cornerstone of all efforts to improve outcomes from sudden cardiac arrest. Debriefing is considered to be an essential component of every cardiac arrest resuscitation effort. Performing quantitative, focused debriefings that look at aspects such as chest compression rate and depth during CPR are critical – studies have shown that high-quality CPR is linked to survival to discharge.
At ZOLL® Medical Corporation, our RescueNet® CodeNet system allows for easy, secure debriefing of resuscitation events. RescueNet CodeNet combines two essential tools for event debriefing and code data documentation – RescueNet CaseReview and RescueNet CodeWriter.
RescueNet CaseReview collects, analyses, and manages code data to improve accuracy and simplify debriefing. With the push of a button, files from ZOLL’s R Series®, X Series®, and ZOLL AED 3® BLS defibrillators can be sent to secure servers using the hospital’s WiFi network. Accurate robust data that’s needed to complete the performance-focused, data-driven debriefing after every adult and paediatric cardiac arrest can be immediately accessed from any device in ZOLL’s browser-based CaseReview software.
The CPR Summary Report, included in CaseReview, provides at-a-glance CPR quality
for individual codes. It can be accessed, saved, emailed, and printed in minutes. It can also be used with code teams immediately after a code
to help facilitate data-driven hot debriefs of the resuscitation event.
CaseReview can also provide trend reports on resuscitation events. These reports can be created through customised tags to review trends by patient type (that is, paediatric versus adult), co-morbidity, department where the code occurred, or even by the time of day or week that the code occurred. A detailed file can be exported into Microsoft® Excel for a deeper dive into data analytics.
Performance-based debriefing should be conducted after sudden cardiac arrest. Debriefing is a critical component for continuous quality improvement (CQI) programs for resuscitation.
‘Hot’ debriefing is usually held very shortly after the resuscitation event and is key to the CQI process. Hot debriefing allows for quick review of actions and interventions while recollections are fresh. This quick, post-event review, conducted with all team members still present when minor details are still maintained, can immediately influence future events.
‘Cold’ debriefing is most beneficial when defibrillator and CPR data are available for review. Cold debriefing allows for comprehensive data availability, adequate time for the debrief review, and valuable discussion time. Debriefing in this group setting allows staff to discuss what occurred and process events, as well as put forward plans for improving protocols.
Documenting a code requires immediate, accurate recording of critical information. Under the stress of the code, capturing key data can be challenging, resulting in inaccuracies due to:
The ZOLL RescueNet CodeWriter documenter is an easy-to-use application for mobile phones or tablets that simplifies code data documentation. Documentation events captured from CodeWriter is merged with the defibrillator data to provide a complete code record.
When used with CaseReview, Codewriter not only captures essential code events, but also helps ensure documentation accuracy with options such as CPR timers, vitals, and key event buttons. Simply launch the app and tap the Start New Code button to get started.
Many factors influence survival from sudden cardiac arrest – co-morbidities such as heart disease, renal insufficiencies, diabetes, and chronic obstructive pulmonary disease (COPD)
are just some of the many factors.
Even when a code team performs at their
very best, there are factors that are too great
to overcome. Knowing where the team was successful during the code and where there was room for improvement leads to better care in
the future.
Copyright ©2019 ZOLL Medical Corporation. All rights reserved. RescueNet, R Series, X Series, ZOLL AED 3,
and ZOLL are registered trademarks of ZOLL Medical Corporation in the United States and/or other countries. Asahi Kasei is a registered trademark of Asahi Kasei Corporation. All other trademarks are the property
of their respective owners.
The novel approach of precision medicine is also reaching the field of mechanical ventilation, with the ultimate aim of customising ventilation and providing patients with the highest quality support. Big steps have been made by manufacturers in the last years, with some new technologies already available for clinical use and which help optimise patient–ventilator interaction.1 However, the greatest effort still comes from clinicians themselves, who should examine the ventilator waveforms in order to detect mismatches between patient and ventilator inspiratory and expiratory times (these phenomena are termed asynchronies). They are observed frequently in ventilated patients and represent a failure in providing them with optimal assistance. Asynchronies have negative clinical consequences such as prolonged mechanical ventilation, difficult weaning, reduced comfort for the patient, increased risk of diaphragmatic damage and potentially increased morbidity and mortality.2–6 Asynchronies can be detected by looking at the ventilator waveforms at the patient’s bedside;7,8 a good knowledge of the phenomenon is therefore essential for diagnosis and correction.
There are a few different classifications of patient-ventilator asynchronies, each of them considering a different aspect of the phenomenon.9,10
Phase
Asynchronies can be classified as inspiratory or expiratory, depending on the neural respiratory phase that is affected. Inspiratory asynchronies are delayed triggering, ineffective effort and autotriggering, whilst expiratory asynchronies are late and early cycling and double triggering.
Relevance
Asynchronies can be classified in major or minor, depending on the type of assistance provided by the ventilator: if there is no correspondence at all between patient’s request and ventilator assistance (that is, the patient starts a breath but the ventilator does not provide any support), the asynchrony is ‘major’, whereas if the ventilator supports the patient in response to his/her request, but the assistance is not appropriate (delayed or not sufficient), the asynchrony is ‘minor’. Mojoli et al pointed out that minor asynchronies might have a greater impact than major ones in ventilated ICU patients.11
Aetiology
Some asynchronies are typically associated with a low patient respiratory drive and/or a too high ventilator assistance (ineffective efforts, delayed cycling, autotriggering, reverse triggering); others are associated with high respiratory drive and low ventilator support, such as early cycling and double triggering.12
The first aspect to consider is the prevalence of asynchronies: they are very common during ventilation, not only in assisted modes but also in controlled modes. In 1997, Chao et al13 observed 200 patients during the weaning from mechanical ventilation and found that 10% of them had ineffective efforts; this phenomenon was associated with prolonged and difficult weaning. This was the first large study focusing on patient–ventilator asynchronies. Following on from this, there was an increasing interest on the subject; other studies confirmed the high prevalence of asynchronies in ICU patients, clarifying their clinical impact as well. Asynchronies started to be considered not only as a cause of discomfort for patients,14 but also as a cause of prolonged mechanical ventilation,6,15 muscle injury, higher sedation requirements,16 and eventually increased mortality.4 Moreover, asynchronies could be involved in long-term neuropsychological effects in patients with respiratory distress.17,18
Clinicians applied different monitoring tools to detect asynchronies (oesophageal pressure, diaphragm electrical activity), and manufacturers produced new modes of ventilation aiming to better fit patients’ requirements (Table 1).
Clinicians progressively learnt how to visually detect asynchronies by looking at ventilator waveforms at the bedside and to adapt ventilator settings breath by breath accordingly, but also realised that the time required for such management was not compatible with everyday clinical practice in the ICU. In fact, patient–ventilator interaction is highly variable among different patients and, in the same patient at different times.4 Moreover, it was suggested that brief clusters of asynchronies and more than average frequency of asynchronies, are associated with poor outcome.19 But it is not feasible to stay at the bedside 24/7 to monitor asynchronies and change the ventilator’s setting according to waveforms. In this context, researchers and manufacturers put their efforts into developing new technologies that are able to replace clinicians in analysing ventilator waveforms and detecting patients’ respiratory activity.1
One can approach asynchronies in two different ways: the first is to rely on software and technologies able to optimise the issue (mainly diaphragmatic electrical activity, other automatic triggering systems available on a few modern ICU ventilators). However, these tools are not always available for every ICU patient. The second approach is based on the observation of ventilator waveforms, the direct recognition of asynchronies and the optimisation of the ventilator settings. Obviously this method is more applicable in the ICU because it does not require special technologies; however, a good knowledge of the most frequent patterns, and of the underlying pathophysiology, is essential to make it efficient.
The waveform method is based on the observation of the standard curves displayed on the ventilator screen (flow and airway pressure), because they are as sensitive and specific as oesophageal pressure, which thus far is considered the gold standard for detection of asynchronies. The method is centred on the identification of the patient’s spontaneous activity.
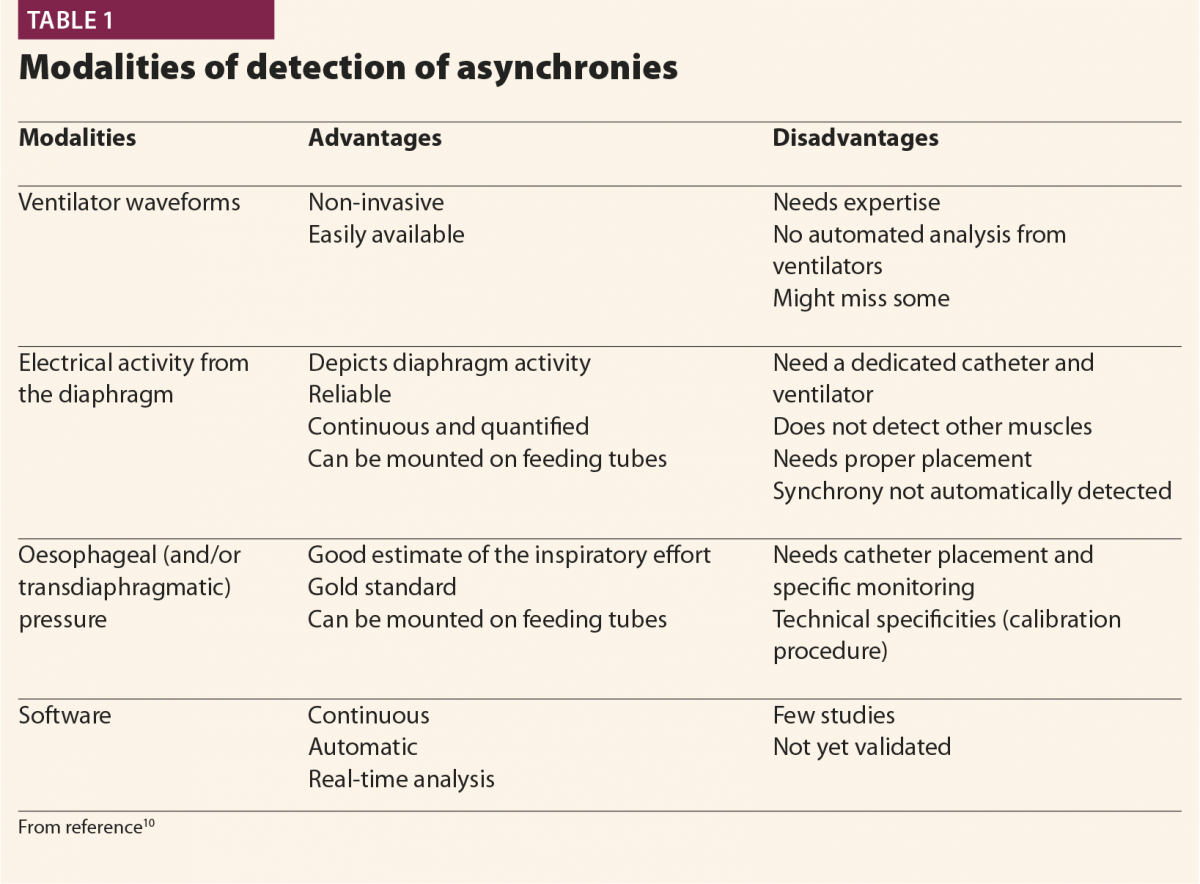
Patient’s inspiration
Typically, when a patient starts a breath, this causes a negative deflection on the pressure curve (Fig.1); on the flow curve, a positive deflection can be detected, even if flow is still negative (Fig.2). Flow and pressure changes correlate with oesophageal pressure, thus they are sufficient to detect a patient’s respiratory activity in most cases.6,7,13
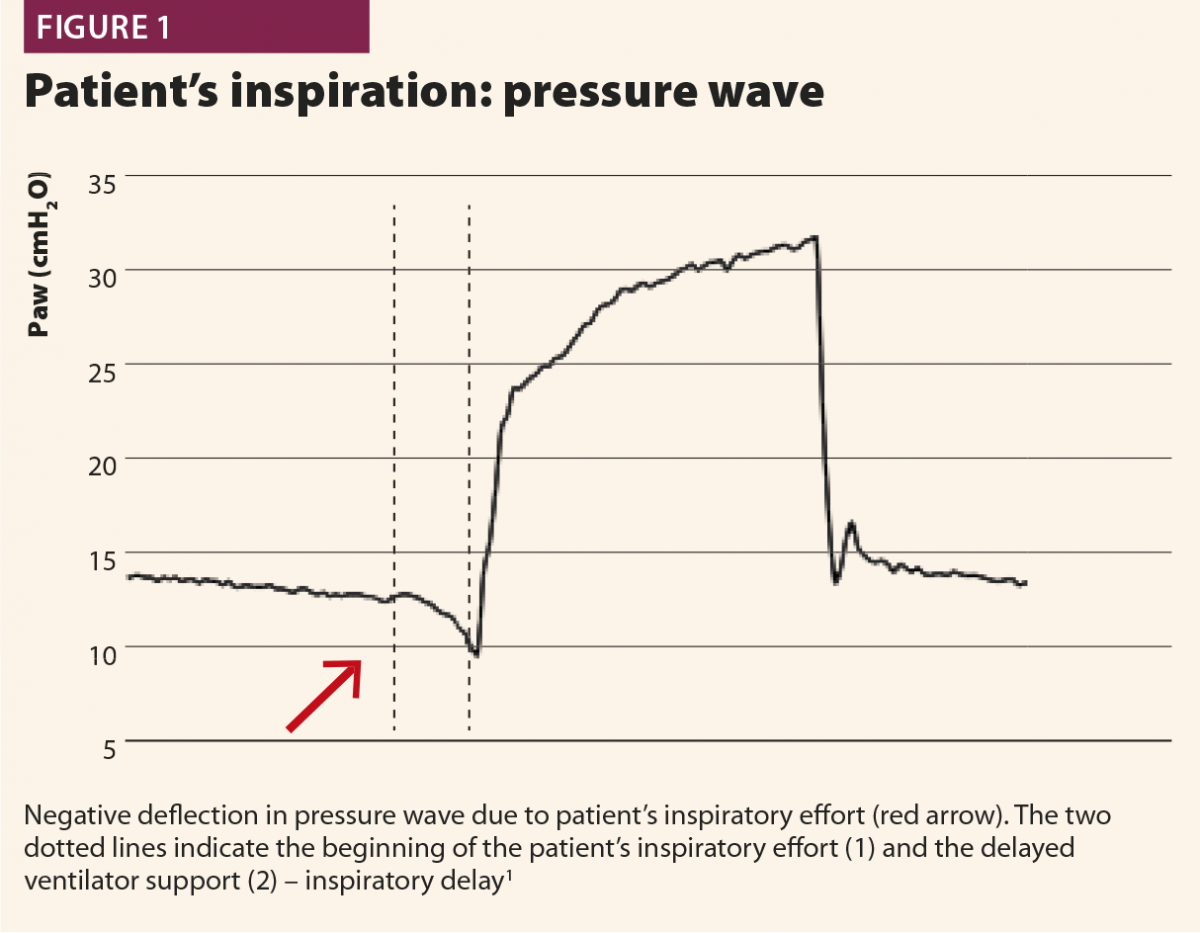
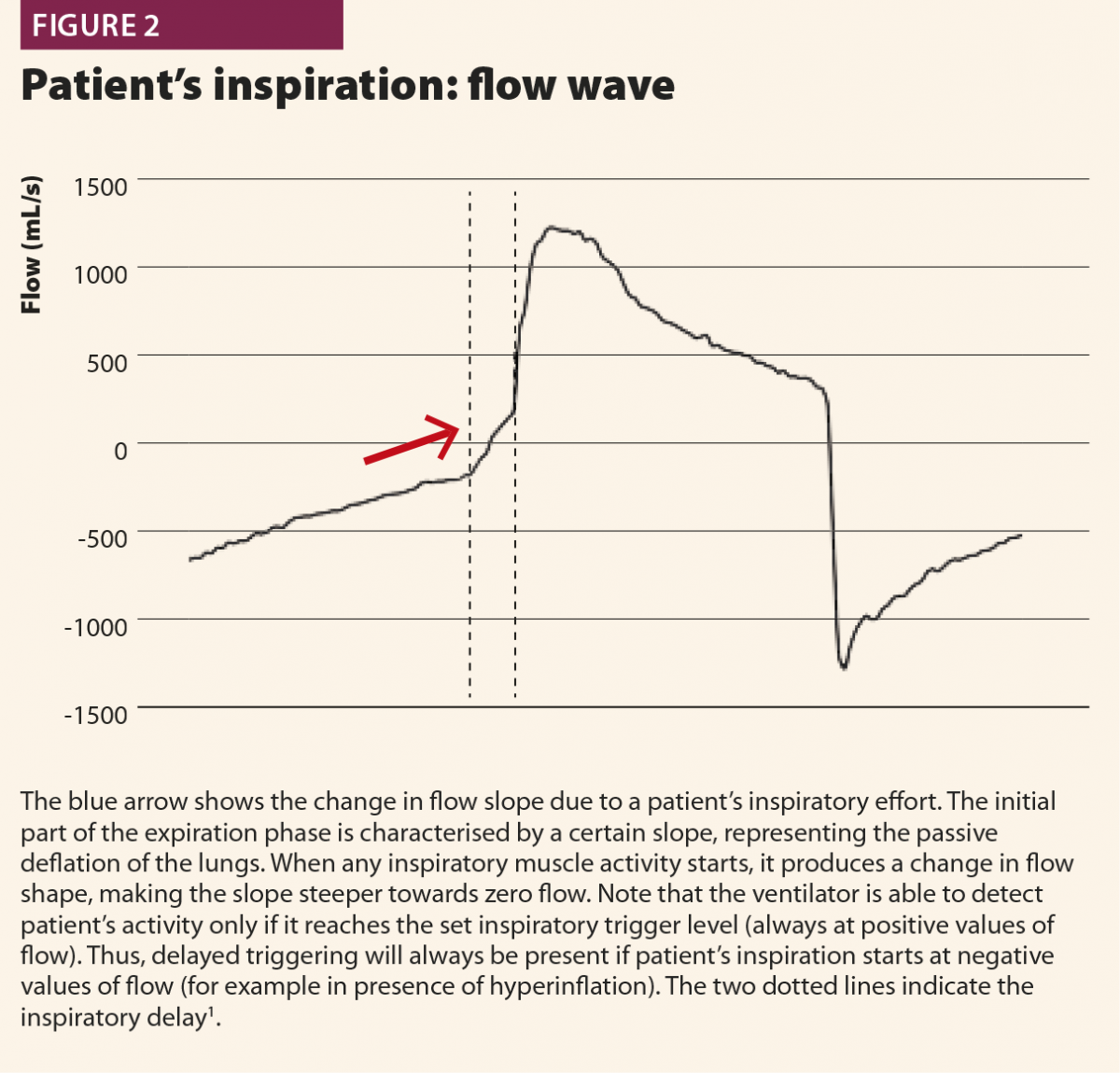
With these simple rules, a patient’s inspiratory activity can be detected even when it is neither detected nor assisted by the ventilator: in other words, ventilator waveforms can reveal a patient’s attempt to trigger the ventilator that does not reach its aim, namely an ineffective effort (Fig.3).
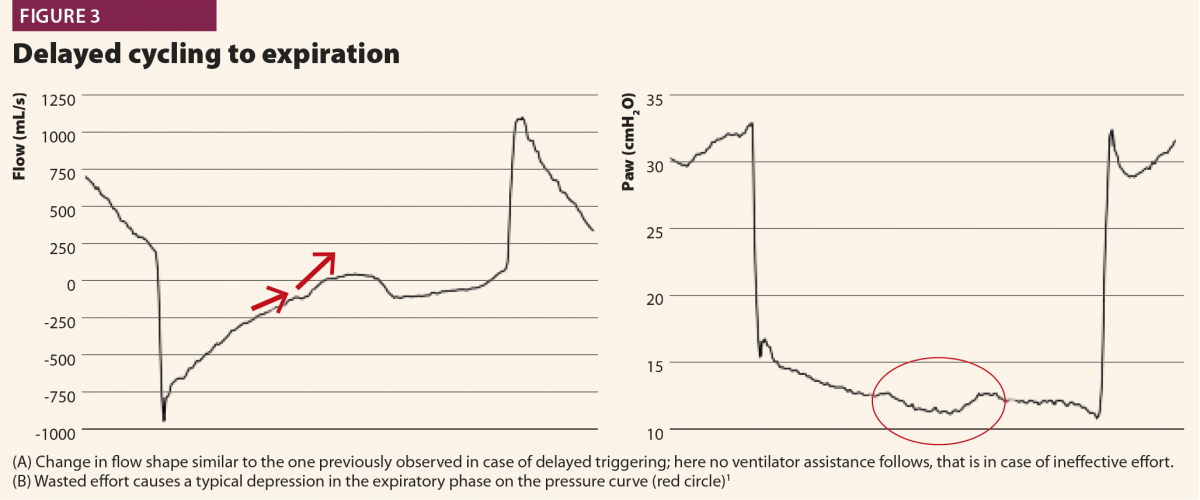
The patient’s start of expiration can also be detected on flow and pressure waves; physiologically, it corresponds to a time point between the nadir of muscular pressure curve and its return to the baseline. This time point varies from patient to patient depending on respiratory mechanics and breathing pattern, but can be conveniently approximated at half relaxation.8
If a muscular pressure curve is not available, indirect signs of relaxation can be detected on flow wave and their appearance varies depending on the assistance given from the ventilator. There are three possible cases1: late cycling, early cycling and optimal cycling.
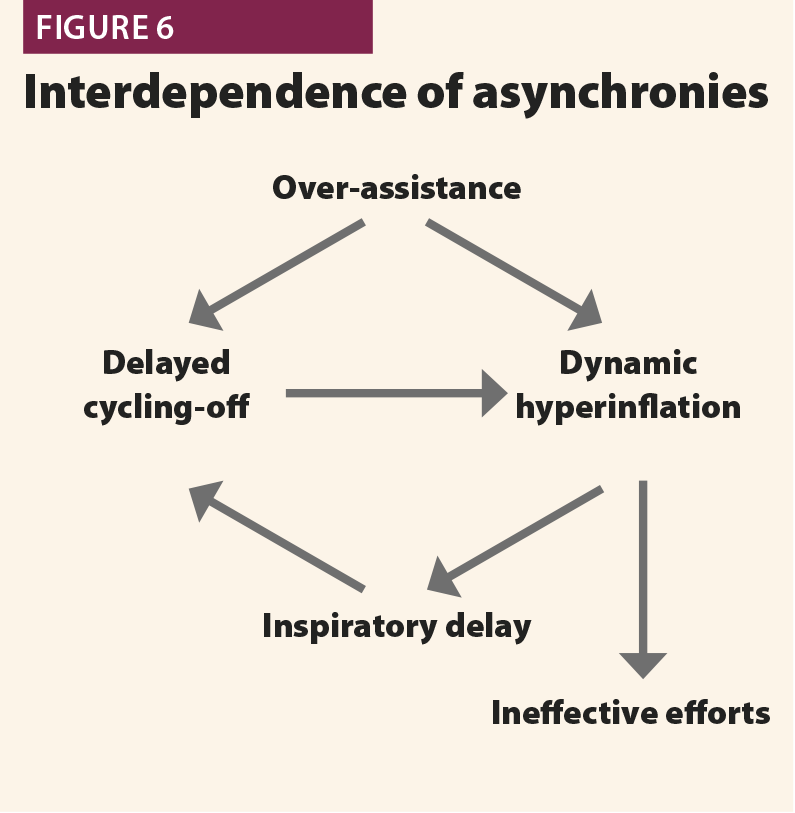
In the first, the machine aerogates air for longer than required; in this case, the patient’s inspiratory muscles will relax during the ventilator’s inspiratory phase, causing a sudden change from fast to slow decrease of inspiratory flow as shown in Fig.4. This often leads to hyperinflation, causing other asynchronies such as ineffective efforts and delayed triggering in the following breaths.20 This phenomenon (called late cycling) is typical of COPD patients and is promoted by a high level of pressure support. Sometimes, patients react to late cycling with active exhalation attemps while the ventilator’s inflation still ongoing, causing a positive deflection on the pressure wave.
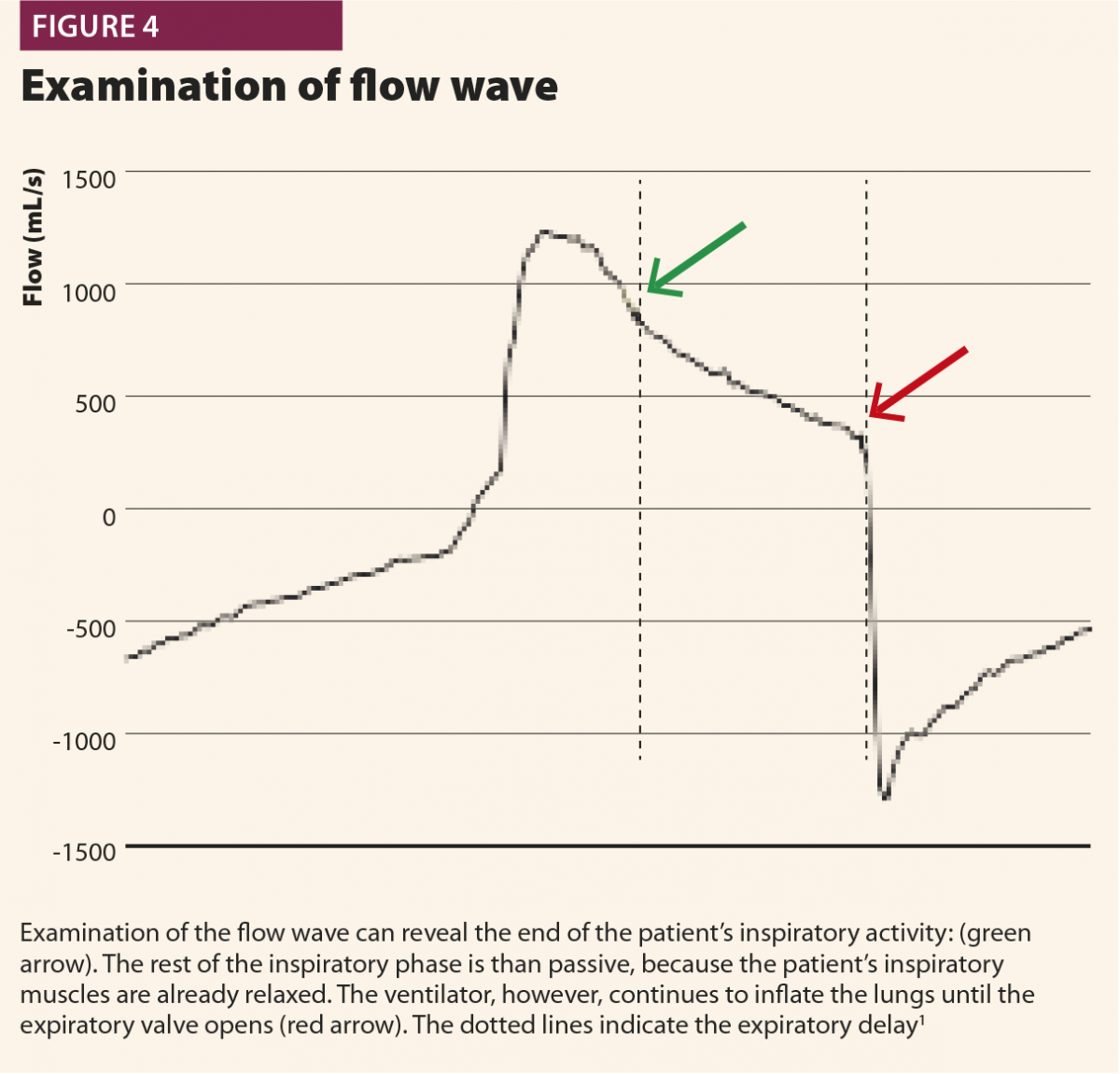
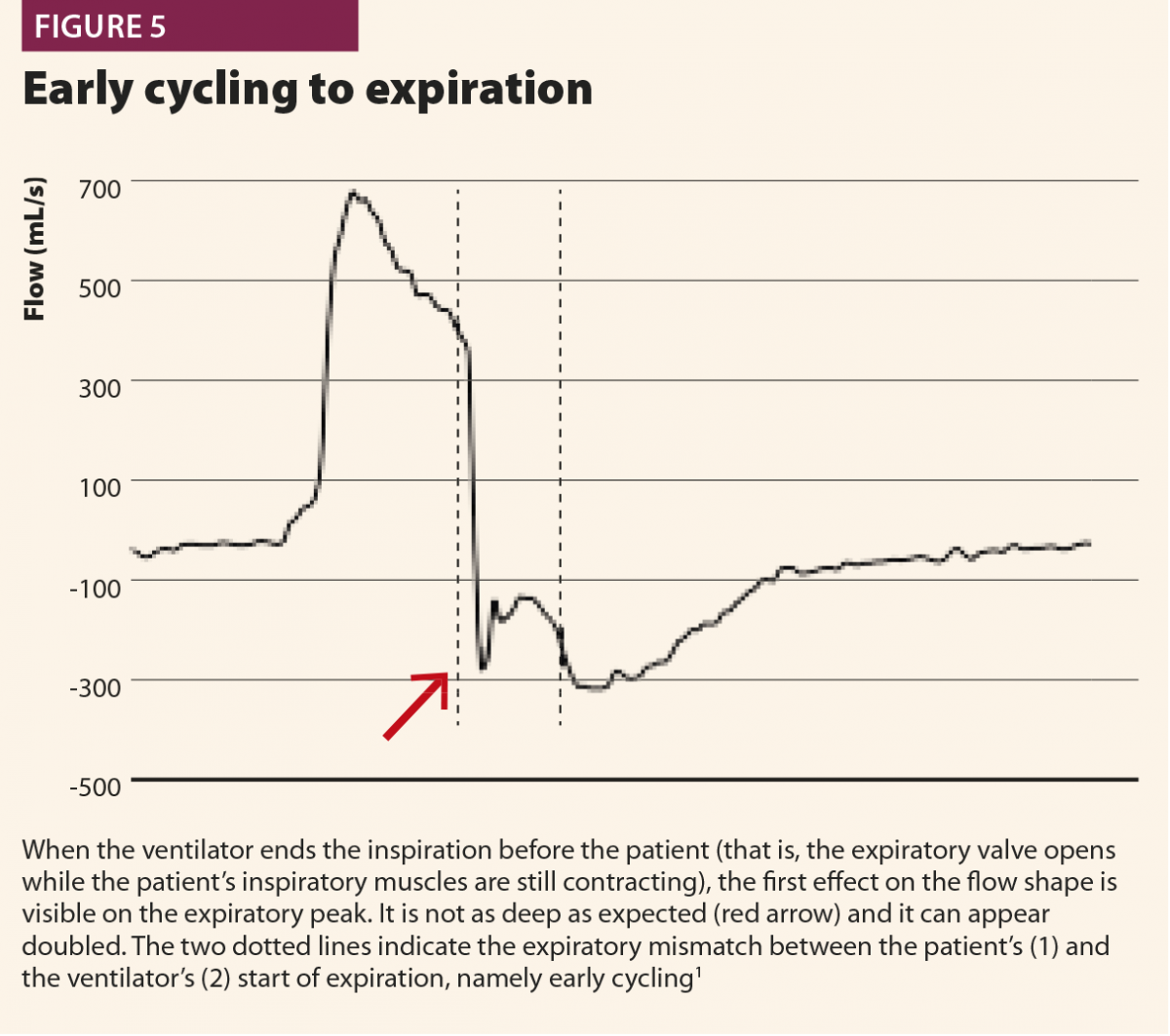
In a second possibility, the ventilator stops aerogating air when the patient’s muscles are still contracting, so expiratory flow is halted by the patient’s inspiratory activity extended after the opening of the expiratory valve, with a typical effect on expiratory peak flow, which appears cut, delayed or ‘doubled’ (Fig.5). Another possible consequence of early cycling to expiration is double triggering. Persistent patient activity after expiratory valve opening can again activate the trigger; thus the ventilator aerogates another breath immediately after the previous one, without a physiological exhalation in between.
In a third case, the ventilator ends its support exactly when the patient’s muscles relax: in this case, inspiratory flow decay becomes faster and faster, directly switching into expiratory flow, with immediate peak and then slow exponential decay.1
Bedside optimisation
Once the clinician has identified the patient’s activity and asynchronies observing the ventilator waveforms, there are a few interventions that can effectively solve the issue. Firstly, any source of external disturbance has to be eliminated (circuit leaks, secretions, circuit occlusions, deconnections), because they can lead to changes in the waveforms and thereby lead to misinterpretation. Second, clinicians have to be aware of the effects of a ventilator’s settings on asynchrony development and act on them appropriately to promote synchronisation.1
Inspiratory trigger
The appropriateness of the inspiratory trigger facilitates the breath initiation and decreases the patient’s work of breathing. Flow trigger is considered better than pressure trigger because it is more sensitive to a patient’s effort and does not require a negative pressure to be produced in the circuit to trigger the ventilator; a little flow entering the inspiratory valve is enough. This leads to more comfortable triggering; however, pressure triggers on modern ventilators have been improved, and the difference between flow and pressure triggers is often very fine.1 As a general rule, trigger sensitivity should be set at the highest value (lowest flow threshold) able to avoid autotriggers, in order to optimise the comfort of the patient.
Pressure support level
Overassistance facilitates asynchronies as well as muscle atrophy very high pressure support levels must be avoided. An excessive pressure support can worsen hyperinflation, leading to difficult triggering (trigger delay and ineffective efforts) and late cycling to expiration.21 When such asynchronies are detected on ventilator waveforms, physicians should consider a decrease of pressure support level.
Ramp
The ramp represents the flow speed to reach the inspiratory peak. As a general rule, for the same sensitivity of the expiratory trigger, a faster ramp makes cycling earlier, whereas a slower ramp makes cycling later. Therefore, a fast ramp can facilitate expiratory synchronisation, especially in COPD patients, whereas a slow ramp increases the time needed to reach lower peak inspiratory flow, thereby favouring late cycling to expiration.
Expiratory trigger sensitivity
The expiratory trigger sensitivity (ETS) is the percentage of the inspiratory flow peak that commands the expiratory valve opening and the cycling to expiration. It can be manually set from minimum values of 5% to maximum of 60–70% of the flow peak; default setting is usually 25% of flow peak.
Setting the ETS appropriately is essential for synchronisation.9,22,23 There is not a ‘one size fits all’ configuration: each patient needs a customised setting, based on the respiratory mechanics and the current respiratory pattern. If the ETS is too low, the ventilator will continue to inflate the patient’s lungs even after the respiratory muscles have relaxed. In other words, a certain amount of the inspiratory phase will be passive, without the participation of the patient’s muscles. By contrast, if the ETS is too high, the ventilator will stop aerogating air even if the respiratory muscles are still contracted: this ‘pliometric’ or ‘eccentric’ contraction can directly damage the diaphragm5,24,25 and can lead to double triggering, breath stacking and lung injury.
An optimised ETS can also positively affect the triggering phase, allowing a physiologic passive exhalation, minimising hyperinflation and ultimately facilitating the trigger for the following breath.
Because COPD patients are prone to late cycling, whereas restrictive patients can experience early cycling, a reasonable approach for initial ETS setting is 25% for patients with normal mechanics (RCexp 0.4–0.8 s), 10% for restrictive patients (RCexp <0.4 s) and 50% for COPD patients (RCexp >0.8 s). Thereafter, the interpretation of bedside ventilator waveforms can be used for fine tuning of ETS.
Sedation
Most of the patients ventilated in assisted modes require some sedation, at least for tube tolerance,26 but excessive sedation is associated with difficult ventilator triggering and with ineffective efforts, mainly for respiratory drive and muscular pressure reduction.15 Optimising sedation is mandatory for correct patient–ventilator interaction management: a lighter sedative plan promotes a patient’s own muscle activity and reduces asynchronies, also allowing
a reduction in pressure support levels.
Automatic monitoring
In the last ten years, big efforts have made in developing software able to detect patients’ respiratory activity and, by computing these data with the ventilator output, toidentify asynchronies. Most of these monitoring softwares were able to work online only for brief periods, usually from minutes to a few hours; in reality, they mainly worked as offline asynchrony analysers, particularly focused on major asynchronies.27–29 The only effective way to monitor patient–ventilator interaction online at the bedside is waveform analysis performed by the expert clinician: indeed, it allows the detection of asynchronies and concurrent optimisation of the ventilator settings. Inevitably, waveform analysis has specific requirements and costs. First, specific training is required, because general clinical expertise and experience do not necessarily correlate with the ability of clinicians in detecting asynchronies by waveform analysis.30–32 Moreover, performing waveform analysis at the bedside is time consuming and requires reoptimisation every time patients change their breathing pattern or their respiratory system resistance and/or compliance for any reason (bronchoconstriction, hyperinflation, increased or decreased pleural effusion…).
In this setting, there is a real clinical need for new technologies that can analyse ventilator waveforms automatically in real time (breath by breath) and continuously (24/7). The ideal software should be able to identify any patient’s respiratory activity, discriminating the beginning and the end of each inspiratory act; it should be able to work online as a trigger to command the inspiratory valve opening and closing according to the patient’s effort. Manufacturers have marketed systems that have been implemented into modern ICU ventilators. They are all promising tools, but not yet validated and no results are currently available to document their performance in improving patient–ventilator interaction.
It is beneficial for ventilated patients to be monitored and optimised in their interaction with the ventilator, and waveform analysis has become essential in administering high quality ventilation. Facing asynchronies requires good knowledge and specific training on the subject.
It also takes time to perform bedside waveform analysis, especially in those cases with difficult patient–ventilator interactions. A possible solution is automation and the market is introducing interesting technologies that will be able to replace the clinicians’ optimisation.
There is a need for further studies to evaluate the performance of new generation triggers in improving asynchronies; in the meantime, the waveform method and the bedside optimisation of ventilator settings remain the most efficient means to manage patient–ventilator interaction.
References
1 Orlando A. How to improve patient-ventilator synchrony. www.hamilton-medical.com/it/dam/jcr:72a82168-9d92-49a6-bb01-0f310dad8fbf… (accessed July 2019).
2 Epstein SK. How often does patient-ventilator asynchronies occur and what are the consequences? Resp Care 2011;56(1):25–38.
3 Sassoon CS. Triggering of the ventilator in patient-ventilator interactions. Resp Care 2011;56(1):39–51.
4 Blanch L et al. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med 2015;41(4):633–41.
5 Nilsestuen JO, Hargett KG. Using ventilator graphics to identify patient-ventilator asynchronies. Respir Care 2005;50(2):202–34; discussion 232–4.
6 Thille AW et al. Patient-ventilator asynchronies during assisted mechanical ventilation. Intensive Care Med 2006;32(10):1515–2.
7 Georgopoulos D, Prinianakis G, Kondili E. Bedside waveforms interpretation as a tool to identify patient-ventilator asynchronies. Intensive Care Med 2006;32:34–6.
8 Mojoli F et al. Is the ventilator switching from inspiration to expiration at the right time? Look at the waveforms! Intensive Care Med 2016;42(5):912–3.
9 Tassaux et al. Impact of expiratory trigger setting on delayed cycling and inspiratory muscles workload. Am J Resp Crit Care Med 2005;172(10):1283–9.
10 Dres M et al. Monitoring patient-ventilator asynchrony. Curr Opin Crit Care 2016;22:246–53.
11 Mojoli F et al. Continuous monitoring of patient-ventilator interaction in ICU patients undergoing prolonged mechanical ventilation. Intensive Care Med 2014;40.
12 Murias G et al. Patient-ventilator asynchrony. Curr Opin Crit Care 2016;22:53–9.
13 Chao D et al. Patient-ventilator trigger asynchrony in prolonged mechanical ventilation. Chest 1997;112:1152–9.
14 Kachmarek RM et al. Assisted mechanical ventilation: the future is now! BMC Anesthesiol 2015;15:110.
15 De Wit M et al. Observational study of patient-ventilator asynchrony and relation to sedation level. J Crit Care 2009;1:74–80.
16 Chanques G et al. Impact of ventilator adjustments and sedation-analgesia practices on severe asynchronies in patients ventilated in assist-control mode. Crit Care Med 2013;41(9):2177–87.
17 Evans KC et al. BOLD fMRI identifies limbic, paralimbic and cerebellar activation during air hunger. J Neurophysiol 2002;88(3):1500–11.
18 Huang M et al. Psychiatric symptoms in acute respiratory distress syndrome survivors: a one-year national multicenter study. Crit Care Med 2016;44(5):954–65.
19 Vaporidi K et al. Clusters of ineffective efforts during mechanical ventilation: impact on outcome. Intensive Care Med 2017;43(2):184–91.
20 Kachmarek et al. Cycle asynchrony: always a concern during pressure ventilation. Minerva Anestesiol 2016;82(7):728–30.
21 Thille AW et al. Reduction of patient-ventilator asynchrony by reducing tidal volume during pressure support ventilation. Intensive Care Med 2008;34(8):1477–86.
22 Chiumello D et al. Effects of different cycling-off criteria and positive end-expiratory pressure during pressure support ventilation in patients with chronic obstructive pulmonary disease. Crit Care Med 2007;35(11):2547–52.
23 Hoff FC et al. Cycling-off modes during pressure support ventilation: effects on breathing pattern, patient effort, and comfort. J Crit Care 2014;29(3):380–5.
24 Gea J et al. Modifications of diaphragm activity induced by midline laparotomy and changes in abdominal wall compliance. Arch Broncon 2009;45(1):30–5.
25 Devor ST et al. Regeneration of new fibers in muscles of old rats reduced contraction-induced injury. J Appl Phys 1999;87(2):750–6.
26 Vaschetto R et al. Effects of propofol on patient-ventilator synchrony and interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med 2014;42(1):74–82.
27 Younes M et al. A method for improving patient-ventilator interaction. Intensive Care Med 2007;33:1337–46.
28 Blanch L et al. Validation of the Better Care System to detect ineffective efforts during expiration in mechanically ventilated patients: a pilot study. Intensive Care Med 2012;38(2):240–7.
29 Sinderby C et al. An automated and standardized neural index to quantify patient-ventilator interaction. Crit Care 2013;17:R239.
30 Colombo D et al. Efficacy of ventilator waveforms observation in detecting patient-ventilator asynchrony. Crit Care Med 2011;39(11):2452–7.
31 Ramirez I et al. Ability of health care professionals to identify patient-ventilator asynchrony using waveform analysis. Resp Care 2017;62(2):144–9.
32 Prinianakis G et al. Effect of the flow waveform method of triggering and cycling on patient-ventilator interaction during pressure support. Intensive Care Med 2003;29(11):1950–9
End of life care (EoLC) is defined as enabling and supporting the palliative care needs of both patient and family to be identified and met throughout the last phase of life and into bereavement. It includes management of pain and other symptoms and provision of psychological, social, spiritual and practical support.1 Few would dispute the importance of EoLC for patients and their families, but it is clear from the literature that EoLC for older patients in emergency departments (ED) is not always as good as it needs to be.2
Indeed, Atul Gawande, the physician and author, believes our reluctance to honestly examine the experience of aging and dying has increased the harm we inflict on people denying them the basic comforts they most need.3
This is a sad indictment but such is the paradox of modern medicine that more people are living longer, often with chronic disease which, for many, results in frequent admissions to the ED. Older patients are often referred to the ED from nursing homes and their own homes when staff or family feel unable to cope with increasing severity of symptoms; this happens even where advanced directives are in place.4 With demand and need for inpatient beds far outweighing supply in most parts of the world, crowding in EDs is commonplace and sadly, but inevitably, some patients (particularly older patients) will end their days in the ED. It behoves us as caring professionals to urgently rethink our response to the needs of older patients dying in the ED so we are not denying them comfort and dignity in their last hours.
There is a perception among ED professionals that the ED is “the wrong place to die”; this perception is understandable because the ED is traditionally designed to save lives, the focus being on saving lives rather than palliation.5 Nonetheless in the current landscape this thinking is outdated and serves as both an excuse and an impediment to improving end of life care in the ED. Some ED physicians have expressed concerns over a lack of training and a lack of skills in caring for patients at the end of life.6–9 Others have described helping someone die in conditions of comfort, dignity and respect as being one of the most gratifying clinical experiences, acknowledging that doing this in the ED is not easy.10 Research suggests that nurses overall are comfortable providing EoLC in the ED but often grapple with inadequate staffing levels and the competing pressures of the ED, alongside delivering the care the older patient needs and deserves and the emotional labour this appropriates.11,12 It would appear, however, that this professional view of the ED as being ‘the wrong place to die’ is not at altogether at odds with public perception and aspiration. Research globally has found that most people, when asked, would prefer to die in their own home surrounded by friends and families; however, most, particularly in the developed world anticipate they will die in hospital.13,14
Delivering good EoLC in the ED is achievable and irrespective of constraints older patients and their families are deserving of privacy, dignity and compassion at the most vulnerable point in their lives and we have a duty to try our utmost to achieve this.
Central to good EoLC is having respect for the dignity and autonomy of the person; this ethos is enshrined in the 1948 Universal Declaration of Human Rights.15 Autonomy as a concept is derived from the ancient Greeks. More recent concepts of autonomy are philosophical products of the Enlightenment and the thinking of philosophers such as John Stuart Mill and Immanuel Kant, the latter proposing the inviolability and intrinsic nature of dignity.16,17
Respecting patient dignity and autonomy means listening and involving patients in decisions about their care. This can take time and calls for what one physician referred to as ‘doing sit down medicine in a stand up place’.18 Time is a precious commodity that is often in short supply in EDs but short-changing older patients and their relatives in terms of time at the point of maximum vulnerability in their lives is not an option.
This need for palliative and EoLC skills for ED clinicians has been recognised by emergency medicine colleges and associations globally and guidelines exist to support ED clinicians, all of which emphasise the importance of respect for the dignity and autonomy of the person.19–21
In the UK in 2013, the Leadership Alliance for the Care of Dying People, published two documents: One Chance to Get it Right and Priorities of Care for the Dying Person,22,23 which set out the approach to caring for dying patients under five priorities. These are:
Recognising dying is an essential clinical skill but nonetheless challenging for most physicians, especially in the ED where there might be no previous knowledge of the patient or his/her medical history.24 Patients must be assessed by a doctor competent to judge whether the patient’s condition is treatable or whether death is likely in the next few hours or days.22,23 Physicians need to be mindful that initiating end of life talks in the ED can be upsetting for patients and families. Finding the right words calls for patience and understanding; privacy for the patient and family must be assured. Being frank and honest with the family and carers is important. These conversations can be very emotional, thus using clear plain language, avoiding euphemisms to minimise any misunderstanding, is essential.
Poor communication at the outset can intensify distress for the patient and carers, and can be the source of difficulty for subsequent care givers and sometimes the source of a complaint.25 Older patients feel vulnerable and are fearful of being alone in the ED, and staff need to be mindful of this.26 Ensuring patients and their family/carers know the name of the doctor and nurse caring for them helps minimise this abject sense of loneliness. Listening actively is key to establishing good rapport. Allow the patient time to talk if he/she can. Remember, patients with cognitive impairment may be slow to answer.
A good preamble is to ask the patient or carers what they already know about their illness; this gives the patient and/or carers ownership of the conversation and can often be quite insightful. Asking the patient about their fears, their goals and what they would like to have done for them will give the physician the way to broach the issue of resuscitation if appropriate.
Responsinf to patient and carer emotions is particularly pertinent in EoLC. As the emotional burden of EoLC and the skills to respond has been cited as a barrier by staff, this could be the right time to involve the palliative care team for added support and advice.
Do Not Attempt Resuscitation (DNAR) orders have had their uses in recognising the limited value of some medical interventions and treatments for older patients with terminal medical conditions, have always been fraught with misunderstanding and ethical difficulties.27 The European Convention on Human Rights mandates that physicians involve patients in making DNAR decisions.28 Physicians should be aware that such conversations can be received by patients and carers with unease and suspicion of DNAR as a withdrawal of treatment. This concern has some veracity as there is evidence that suggests in-hospital mortality is higher in patients with DNAR orders than for those with similar comorbidities and severity of illness without a DNAR in place.28,29 The physician needs to reassure the patient and carers that DNAR only applies to CPR, and not medication, comfort measures or general care.
Nonetheless, concerns about a DNAR and the associated difficulties have led to calls for a change of approach from DNAR to goals of care or universal forms of treatment.29,30 Goals of care are a multidisciplinary approach where the starting point is about what can be done rather than what cannot. It is quite a different approach, whereby the aim is to decide treatment choices and care needed.29
Unlike DNAR, the goals of care model places greater emphasis on emotional support for patients and their families and is an altogether more positive but realistic approach.29 Patients may be more receptive to this approach and physicians may be more inclined to initiate such conversations with patients on these terms. The Universal Form of Treatment Options (UFTO) is a similar concept which was developed with patients, doctors and nurses as an alternative approach to resuscitation decisions. In this approach, resuscitation decisions are contextualised within overall goals of care.30
Where patients lack capacity to make decisions about CPR or goals of care, the physician must ask whether there is a Lasting Power of Attorney or an Advance Directive relating to CPR in place. In the absence of either, the physician needs to consult those close to the patient, providing it is appropriate to do so. Those consulted must be advised that the overall decision lies with the clinical team.31
Supporting the older patient and family as death is imminent is one of the many privileges of our roles and the very cornerstone of caring but it can be a challenge to achieve in a crowded department. Having a dedicated room in the ED, or at least redesigning the physical space of the ED to accommodate EoLC with allocated nurse staffing, is advisable and preferable to transferring them to a ward as death is imminent.32
Having dedicated space would also encourage staff to reorient their thinking from the ED being the ‘wrong place’ to die. Ensuring privacy and explaining what to expect as death approaches is important in allaying anxiety for family and carers. Clinicians should never underestimate the power of a kind word or gestures of empathy and the enormous difference these can make to the grieving process.
Care needs to be holistic. Common symptoms requiring treatment in the older patient include pain, difficulty in breathing, noisy respiratory secretions, nausea and vomiting, dysphagia, incontinence and anxiety.29,30
Dyspnoea is experienced by many older patients with end-stage cardiac and respiratory conditions. This, and intractable pain, is often the crisis point for patients and families who feel unable to cope with the increasing severity of symptoms and need the reassurance and support of the ED. Although dyspnoea is a physical sign, it can also be a manifestation of emotional distress.
Morphine, recommended by the World Health Organization, is the most frequently used drug for the symptomatic management of dyspnoea and is included in their evidence-based list of essential medicines in palliative care, which was updated in 2013. Morphine has the advantage of also relieving pain and anxiety but it is also important to consider other likely causes of such distress.
Nursing interventions such as regular repositioning as well as personal hygiene and mouth care are fundamental for patient comfort. Interventions such as intravenous cannulas or urinary catheters must be justified for patient comfort and clearly explained to the patient, their family and carers. Clinicians should not overlook the spiritual component of death and dying. In our increasingly diverse society, death and dying are understood and experienced differently depending on cultural and religious meanings.
It is important to be culturally sensitive to diverse rituals, fulfilling the wishes of the patient and family as far as possible and reasonable should be the goal.
Where there are language barriers, best practice advocates the use of interpreters, but older patients might be unable to relate to an interpreter and prefer to have their families interpret for them. Families may also find the presence of an interpreter intrusive and quite inappropriate in the private space of their dying loved one. Staff should offer to call the Chaplin/Priest/Imam/Rabbi or relevant spiritual leader at any time if the patient desires.
Delivering good EoLC for the older patient in the ED is an increasing imperative as it is inevitable that more older people will spend their last hours in the ED. An acceptance of EoLC is an important function of the ED. Developing palliative and EoLC skills is essential to ensure that patients die with dignity and that the memory for families and carers is not marred by experiences of poor care.
Having a designated space that provides privacy and dignity is fundamental but this also requires adequate staff who are trained in EoLC to support the patient and family. Multidisciplinary training and closer working with in-hospital and community palliative care teams should be encouraged. A goals-of-care approach will reassure patients and their families that they are being cared for. Small changes can make a big difference, and with a different ethos and adequate resources, we can ensure that the ED is not ‘the wrong place to die’ and that older patients do not suffer unnecessarily in their last hours.
References
1 Council of Europe. Committee on Bioethics (DH-BIO) Guide on the decision-making process regarding medical treatment in end of life situations 2014. www.coe.int/en/web/bioethics/end-of-life (accessed August 2019)
2 Cooper E et al. Palliative care in the emergency department: A systematic literature qualitative review and thematic synthesis. Palliat Med 2018;32(9):1443–54.
3 Gawande A. Being Mortal and What Matters in the End. 2014.
4 Stephens C et al. Provider perspectives on the influence of family on nursing home resident transfers to the Emergency Department: Crisis at the end of life. Curr Gerontol Geriatr Res 2015;2015;2015:893062.
5 Bailey C. Murphy R, Porock D. Dying cases in emergency places: Caring for the dying in emergency departments. Soc Sci Med 2011;73:1371–7.
6 Giscondi MA. A case for education in palliative and end of life care in Emergency Medicine. Acad Emerg Med 2009;16(2):181–3.
7 Jelinek GA et al. Better pathways of care: suggested improvements to the emergency department management of people with advanced cancer J Palliat Care 2014;30(2):83–9.
8 Shearer M, Ross-Adjie L, Rogers JR. Understanding emergency department staff needs and perceptions in the provision of palliative care. Emerg Med Aust 2014;26:249–55.
9 Fassier T et al. Who am I to decide whether this person is to die today? Physicians’ life-or-death decisions for elderly critically ill patients at the Emergency Department–ICU interface: A qualitative study. Ann Emerg Med 2016;68(1):28–39.
10 Marck CH et al. Care of the dying cancer patient in the emergency department: findings from a National survey of Australian emergency department clinicians. Int Med J 2014;44(4):362–8.
11 Wolf L et al. Exploring the management of death: Emergency nurses perceptions of challenges and facilitators in the provision of end of life care in the emergency department. J Emerg Nurs 2015;41:e23–e33.
12 Bailey C, Murphy R, Porock D. Professional tears: developing emotional intelligence around death and dying in emergency work. J Clin Nurs 2011;(23-24):3364–72.
13 Kaiser Family Foundation. Views and Experiences with End-of-Life Medical Care in Japan, Italy, the United States, and Brazil: A Cross-Country Survey. The Economist; April 2016.
14 Gomes B et al. Preferences for place of death if faced with advanced cancer: a population survey in England, Flanders, Germany, Italy, the Netherlands, Portugal and Spain. Ann Oncol 2012;23:2006–15.
15 Universal Declaration of Human Rights 1948. www.un.org/en/universwl-declaration-human-rights (accessed August 2019).
16 Kant I. In: Groundwork for the Metaphysics of Morals (translated by Wood A) New Haven, Yale University Press. 2002:2–58.
17 Haugen HM. Inclusive and relevant language; the use of concepts of autonomy, dignity and vulnerability in different contexts. Med Health Care Philosophy 2010:13(3)203–13.
18 Adler E, Qui Q. Performing sit down medicine in a stand-up place: is it time for palliative care in the emergency department? Emerg Med J. 2018;35(12):730–1.
19 Royal College of Emergency Medicine. End of life care for adults in the emergency department. www.rcem.ac.uk/docs/College%20Guidelines/5u.%20End%20of%20Life%20Care%20for%20Adults%20in%20the%20ED%20(March%202015).pdf (accessed August 2019).
20 European Society for Emergency Medicine. European recommendations for end of life care for adults in departments of emergency medicine. https://eusem.org/wp-content/uploads/2017/10/EuSEM-Recommendations-End-o… (accessed August 2019).
21 American College of Emergency Physicians. Palliative Medicine in the Emergency Department. www.acep.org/how-we-serve/sections/palliative-medicine/palliative-medici… (accessed August 2019).
22 Leadership Alliance for the Care of Dying People. One Chance to Get It Right. https://assets.publishing.service.gov.uk/government/uploads/system/uploa… (accessed August 2019).
23 Leadership Alliance for the Care of Dying People. Priorities of care for the dying person. www.nhsemployers.org/news/2014/06/leadership-alliance- (accessed August 2019)
24 Taylor P, Dowding D, Johnson M. Clinical decision making in the recognition of dying: qualitative interview study. BMC Palliative Care 2017. https://bmcpalliatcare.biomedcentral.com/articles/10.1186/s12904-016-0179-3 (accessed January 2019)
25 Whitehouse S. We need to talk about death. Complaints about end of life care 2013. www.medicalprotection.org/uk/casebook.
26 Arendt G et al. Preferences for the emergency department or alternatives for older people in aged care: A discrete choice experiment. Age Ageing 2017;46(1):124–9.
27 Fritz Z, Fuld J. Ethical issues surrounding do not attempt resuscitation orders: decisions, discussions, and deleterious effects. J Med Ethics 2010; 36(10):593–7.
28 Chen JL et al. Impact of Do Not Resuscitation Orders on quality of care performance measures in patients hospitalized with acute heart failure. Am Heart J 2008;156:78–84.
29 Arabi YM, Al Sayyari A, Moamary MS. Shifting paradigm: from “No Code” and “Do-Not- Resuscitate” to Goals of Care Policies. Ann Thoracic Med 2018;2(25):67–71.
30 Fritz Z, Fuld JP. Development of the Universal Form of Treatment Options (UFTO) as an alternative to Do Not Resuscitation (DNACPR) orders: a cross-disciplinary approach. J Eval Clin Pract 2013;2:109–17.
31 Sokol DK. Cautionary tales about DNACPR. BMJ 2016;352.
32 Beckstrand RL et al. Emergency nurses suggestions for improving end of life care obstacles. J Emerg Nurs 2012;38(5):e7–e14.
Results presented at the Presidential Symposium of the IASLC 2019 World Conference on Lung Cancer have shown improved overall survival with durvalumab (brand name Imfinzi) in small cell lung cancer.
The Phase III CASPIAN trial showed significantly improved overall survival in patients with previously-untreated extensive-stage small cell lung cancer. Durvalumab in combination with four cycles of standard-of-care (SoC) chemotherapy (etoposide with either cisplatin or carboplatin) demonstrated a statistically-significant and clinically-meaningful improvement in OS versus SoC comprising up to six cycles of chemotherapy and optional prophylactic cranial irradiation (PCI).
The risk of death was reduced by 27% (HR 0.73), with median OS of 13.0 months for durvalumab plus chemotherapy versus 10.3 months for SoC. Results showed a prolonged OS benefit with an estimated 33.9% of patients alive at 18 months following treatment with Imfinzi plus chemotherapy versus 24.7% of patients following SoC.
Across all efficacy endpoints, benefits were observed in patients treated with durvalumab plus chemotherapy versus SoC. Results showed a significantly higher progression-free survival rate at 12 months (17.5% vs 4.7%), a 10.3% increase in confirmed objective response rate (67.9% vs 57.6%), and improved duration of response at 12 months (22.7% vs 6.3%).
José Baselga, executive vice president, oncology R&D, said: “We are encouraged to see more than a third of small cell lung cancer patients treated with Imfinzi plus chemotherapy alive at the 18-month landmark, which is remarkable given the aggressive nature of the disease. It is also noteworthy that these results may enable physicians to choose Imfinzi in combination with either cisplatin or carboplatin chemotherapy backbones. We look forward to working with regulatory authorities to bring Imfinzi to patients with small cell lung cancer around the world as soon as possible.”
Luis Paz-Ares, MD, PhD, chair, medical oncology department, Hospital Universitario Doce de Octubre, Madrid, Spain, and principal investigator in the Phase III CASPIAN trial, said: “Patients have had limited treatment options for small cell lung cancer, a devastating disease where the five-year survival rate has been as low as 6%. The significant survival benefit demonstrated with Imfinzi combined with only four cycles of a choice of chemotherapy compared to a robust control arm, provides evidence and hope of a new treatment option for these patients.”
11th September 2019
Cancer Research UK states that between 2014 and 2016, 363,484 new cases of cancer were reported and, with year-on-year increases, these drugs will only become more commonly used.
Furthermore, in 2018, a National Health Service (NHS) commissioned report recorded an increase in demand for aseptically prepared products of around 5% per year.1
Demand is likely to grow further with increased usage of advanced therapy medicinal products (ATMPs), growth in clinical trials, and potentially the necessity to address the sizeable unmet need for central intravenous additive services and monoclonal antibodies.
To compound the issue, demand for aseptic medicines is predicted to continue to increase in line with global drug expenditure, and in particular, injectable medicine, for which sales are growing at 7.3% compound annual growth rate (CAGR).2
Currently, the NHS relies on commercial suppliers for at least a third of aseptic compounding needs with 180 known NHS aseptic facilities in England (excluding radiopharmacies) and 17 sites that completely outsource production. The 2018 report suggests that the reason for this reliance on commercial suppliers is that ‘Trusts who have chosen to outsource did so for cost efficiency, investment, or capacity reasons.’3
Apart from demand, the NHS is contending with several other “compounding” issues. The first is that aseptic preparation is labour intensive and many facilities do not have the numbers of staff required to keep up with demand.
The second is that in-house aseptic preparation is expensive because of the need to buy, maintain and replace equipment.
Lastly, and most importantly is the issue of safety. The workers who compound hazardous drugs, of whom the European Commission state there are 20 million European healthcare workers, are at risk of negative health outcomes.4 Studies from the National Institute of Occupational Safety and Health (NIOSH) have shown that these can include abdominal pain, nausea, vomiting, diarrhoea, coughing, facial flushing, hair thinning, hair loss, dermatitis, irritation of skin and eyes, irritation of mucous membranes, menstrual cycle disruption, and foetal loss, and even some forms of cancer.5
With all of these issues facing the NHS, there is a real need to look at new ideas and alternative solutions. One potential approach that could solve all of these challenges would be to move to automation for hazardous drug compounding.
The most important advantage of automation is that it can keep healthcare workers safe as robotic automation systems can physically handle the drugs during the compounding process.
To further increase safety and contain hazards, compounding systems that can incorporate the use of a closed system transfer device (CSTD) would provide a key layer of protection for staff, which must be of paramount importance. It offers the highest protection level, which is prevention of exposure to hazardous drugs.
Robotic automation systems can also compound drugs at a much faster rate compared with manual compounding. This could potentially free-up members of staff to attend to other important tasks while still ensuring that the drugs that are needed are being compounded, while also lessening the element of human error by preventing dosage and medication errors.
The fact that drugs could be compounded at a faster rate could also reduce the overall cost that facilities incur for paying for the machines and their maintenance. Fewer machines would be required and as a consequence, upkeep and repairs would be lower than they currently are – maintenance alone can cost tens of thousands of pounds each year.
Equally, one central service could produce drugs for several facilities, allowing for sharing of the overall cost. While compounding centres stand to gain significantly from automation technologies, it is crucial that they have in place contingency plans, as well. For example, if the automation system is down, the centre must have sufficient personnel to handle the compounding work in a safe environment. Any facility considering a move to automation should ensure that they also develop protocols to handle unexpected mishaps with these systems.
Notwithstanding the need for contingency planning, the use of automation seems to be the natural next step forward for the safer and more efficient compounding of hazardous medicines. Many other industries with mission critical applications have embraced robotic automation, allowing them to flourish and develop. With the latest challenges in meeting ever increasing demand and improving safety, it would seem like the opportune time for chemotherapy compounding followed suit and embrace automation. The technology is now available, and it is up to facilities to take full advantage of it to improve the quality, efficiency and safety of hazardous drug compounding.
Dr Paul JM Sessink studied organic chemistry and toxicology at the University of Nijmegen in The Netherlands, where he also completed a PhD degree in Medical Sciences.
He is the founder of Exposure Control, a consulting firm for the monitoring of hazardous drugs and is co-author of approximately 40 scientific publications regarding environmental and biological monitoring of occupational exposure to cytotoxic drugs.
References
1 Source: NHS stakeholder interviews; Aseptic Facility data collection Jan 2018.
2 NHS Improvement (March 2018). Pharmacy Aseptic Services Review Summary of Key Findings.
3 Ibid.
4 European Federation of Nurses Associations, European Expert Group on Hazardous Drugs, May 8, 2017.
5 Centers for Disease Control and Prevention, 2017; Centers for Disease Control and Prevention, 2019
The dosing of chemotherapy agents involves a delicate balance between the desired efficacy and the drug’s acceptable toxicity. Traditionally, the doses of anticancer drugs were calculated according to body weight or surface area, but in 2018, the National Institute for Health and Care Excellence (NICE) released a position statement supporting dose standardisation, or dose banding, for intravenous chemotherapy drugs for adults with cancer. This standardisation and optimisation of doses in oncology brings benefits to hospitals, as well as health care providers and patients.
The objective of having standardised doses in the treatment of cancer is to facilitate the preparation of intravenous chemotherapy agents within hospital pharmacy aseptic units, and also the administration in the oncology ward. The chemotherapy schedule for a patient with cancer is often complex and involves multiple drugs and dose adjustments as part of a regimen that is tailored to each individual case. With dose standardisation, the doses of intravenous anticancer drugs are approximated to pre-determined standard doses and clustered in dose ranges or bands, which can significantly reduce preparation time, mitigate the risk of calculation errors, and reduce waste resulting from left-over drugs.1
The chemotherapy drugs purchased in bulk by a particular hospital can then be standardised to match the recommended pre-defined dose bands and prepared as single-serve doses in advance, provided these drugs show long-term physicochemical stability after compounding (that is, greater than 30 days). Alternatively, pre-compounded products can be acquired.
This dose standardisation process involves, in general; the approval of basic practices for the definition of dose bands by the local oncology and hematology departments; the approval of national or regional dose banding tables by local formulary committees; the prescription and dispensing of the approved drugs according to the doses listed in these tables; and the establishment of uniform practices for the purchase of ready-to-administer products.1
The role of the pharmacist in the administration of chemotherapy agents is critical, ranging from the verification of the prescription to the preparation of the drug(s) to be administered. The verification of prescriptions, which might involve a specific number and type of checks for each prescription, and the reconciliation of systemic anticancer agents, must be carried out by accredited professionals, according to standards defined by the British Oncology Pharmacy Association (BOPA).2
Prescription errors can be particularly dangerous in this setting. An analysis of prescriptions for chemotherapy agents against BOPA standards in England, Scotland and Wales identified errors in 2.3% of prescriptions during the mandatory prescription verification process, which could potentially result in medical intervention or hospitalisation, or even serious injury or death. In addition to prescription verification, pharmacists are responsible for reviewing all the medicines taken by a patient who is about to initiate chemotherapy in order to minimise the risk of adverse effects of the treatment such as toxicities or reduced efficacy owing to presence of comorbidities or the use of concomitant and/or alternative medicines that can interact with the conventional anticancer agents. Together with nurses trained in oncology, pharmacists can also provide counselling to patients and follow up on the use of any medicines, ensuring adherence to the treatment.2
Given the toxicity of these drugs and the significant investment in time and money from the institutions and staff, it is of utmost importance that all health care professionals involved in chemotherapy patients with cancer adhere to the standards for dose banding defined by their organisations. Regular quality audits should be conducted at all levels of involvement, and any deficiencies should be promptly communicated and corrected.2
Chemotherapy dose bands have been gradually implemented at several hospital trusts in England for the past ten years, although at variable pace. However, some level of heterogeneity has been observed in terms of processes used for dose banding and the drugs included in the bands.
In Scotland, where dose standardisation is now established, 60–70% of all chemotherapy agents are currently being administered according to pre-specified dose bands.1
In order to promote the adoption of dose banding for several intravenous anticancer agents, National Health Service (NHS) England has formed Medicines Optimisation and Chemotherapy Clinical Reference Groups with the aim to uniformise prescription practices across the entire region based on previously approved dose bands. The implementation efforts of these reference groups are being encouraged by NHS England via a Commissioning for Quality and Innovation initiative (CQUIN) scheme, which releases funding upon demonstration of improvements in quality from the participating trusts.1
Currently, there are pre-determined dose bands for 54 chemotherapy agents in England. NHS England’s Medicines Optimisation Intelligence Group is responsible for collecting data to support this implementation, and specific measurement tools and recommendations have been developed by the Chemotherapy Dose Standardisation Steering Group to measure the impact of this initiative. NICE is closely collaborating with NHS England to widen the implementation of dose standardisation for chemotherapy drugs, providing specific guidance on drug sourcing and supply, as well as contracting and tendering. In addition, NICE provides recommendations for the identification of waste during the preparation process, and for the measurement of the impact of dose banding on the patients’ experience with their treatment, as well as on staff satisfaction and the financial impact on Trusts.1
The benefits of chemotherapy dose standardisation are obvious, most notably the time-saving and cost-reducing benefits. In England, the costs incurred by the NHS with chemotherapy amount to approximately £1.5 billion, of which 80% represent anticancer medicines. In addition, these costs seem to grow by approximately 8% every year, which significantly contributes to the financial burden of the health system. With the implementation of discrete dose bands, the administered doses of conventional chemotherapy agents are actually about 6% of the calculated dose for the patient; for biological agents, which are traditionally much more expensive, it is approximately 10%.1
An analysis of costs and parenteral chemotherapy drug use following dose standardisation in a tertiary oncology centre in England showed a reduction in approximately £100,000 per month on 17 dose-banded drugs, despite an increase in the number of prescribed doses during the same period of time. These encouraging results were accompanied by a reduction of approximately 10% in the total workload associated with drug compounding, ultimately increasing the capacity and productivity of the centre’s aseptic compounding unit.3
Dose banding can also reduce patients’ waiting times because the ready-to-use drugs can be administered on any day that fits their schedules. Moreover, this practice allows patients to receive their treatment at facilities closer to their residence, given that no special compounding units are required for drug preparation. From the perspective of health care providers, dose banding results in reductions in the time spent with drug preparation and minimises dose calculation errors. Dose banding also prevents time-consuming changes in prescriptions and allows for a rapid dispensation through the use of pre-prepared doses. Financial efficiency can be further improved by outsourcing standardised pre-filled bags of chemotherapy agents for infusion. For commissioners, the uniformisation of doses at the national or regional level contributes to reduced costs arising from the reuse of doses that are not used due to changes in doses during treatment or due to cancellation of the treatment, and from the reduction in incomplete vial usage during the preparation process.1
In addition to these proven benefits, the available evidence suggests that the use of dose bands does not seem to have a negative impact on the toxicity associated with chemotherapy drugs or on clinical outcomes for patients.1
A retrospective study conducted in France in 2012 to compare the pharmacokinetic profiles of chemotherapy drugs, administered at regular fixed doses and as dose bands, showed no differences in drug exposure between the two dosing approaches.4 Another study in England showed the feasibility of the dose banding strategy for five anticancer drugs in paediatric patients with ages ranging from 1 month to 18 years, based on pharmacokinetic parameters.5
The standardisation of doses of intravenous cytotoxic chemotherapy agents was initially proposed to improve pharmacy capacity and reduce medication errors and wastage. However, further optimisation of the administration of anticancer drugs can potentially contribute to a more efficient oncology unit. In addition to dose bandings, the use of solvents, volume and labelling of chemotherapy agents can also be subject to uniformisation, which can potentially minimise the risks posed by these toxic drugs to both patients and staff. In the future, a partial or full automation of the drug preparation process may represent an advancement in terms of improvements in drug management since the number of patients diagnosed with cancer continues to increase every year. Formal evaluation of the feasibility, consistency, quality control and assurance of validated dose banding procedures in routine practice are also needed in order to demonstrate a reduction of the financial pressure placed on health systems due to non-standardised dosing.
References
1 National Institute for Health and Care Excellence. Chemotherapy dose standardization. February 2018. www.nice.org.uk/advice/ktt22 (accessed February 2019).
2 British Oncology Pharmacy Association. Medicines Optimisation, Safety and Clinical Pharmacy Workforce Plan. January 2015. www.bopawebsite.org/sites/default/files/publications/Clinical_pharmacy_w… (accessed February 2019).
3 Chatelut E et al. Dose banding as an alternative to body surface area-based dosing of chemotherapeutic agents. Br J Cancer 2012;107(7):1100–6.
4 Finch M, Masters N. Implications of parenteral chemotherapy dose standardisation in a tertiary oncology centre. J Oncol Pharm Pract 2018:1078155218812943.
5 White-Koning M. Investigating the potential impact of dose banding for systemic anti-cancer therapy in the paediatric setting based on pharmacokinetic evidence. Eur J Cancer 2018;91:56–67.