This website is intended for healthcare professionals only.
Take a look at a selection of our recent media coverage:
17th September 2019
In England, women are invited for screening for three types of cancer concurrently in their sixties; for the last cervical screen before they exit the programme, for breast screening every three years, and for bowel screening every two years.
This means that an average woman aged 60 can expect to receive five or six cancer screening invitations by the time she turns 65. In England, cancer screening is provided by the NHS free of charge.
In a study published in the Journal of Medical Screening, researchers categorised a sample of just over three thousand eligible women in their sixties according to the last screening round. They looked into women’s participation in all three programmes.
Results showed that:
They also found that general practices with a higher proportion of unemployed patients and a higher number of smokers had a lower rate of take-up of all three screening programmes. Conversely, take-up was more frequent among practices in areas of less deprivation, with a higher proportion of women with caring duties, those with long-term health conditions, and those with a high level of patient satisfaction with the practice itself.
“To lower the chances of dying from certain cancers, it is important for the population to attend all offered screening programmes,” said lead author Dr Matejka Rebolj from King’s College London.
“We know from the official statistics that the majority of women are up to date with breast screening, but this drops to just over 50% when it comes to bowel screening. It is worrying that only a third of women are up to date with all offered cancer screenings and that 10% remained completely unscreened in the last round. Indeed, similar patterns have been reported from other countries too.
“It is crucial for us to look at the take-up rates in certain areas and in certain practices and address women’s preferences for future screening programmes. We need to understand and target specifically those women who obtain some screening, but decide not to take up all the life-saving screening that is offered to them by the NHS. It is important that policy makers now look at these findings to inform what can be done in the future to reduce the significant number of deaths in the over 60-year olds.”
Senior author Professor Stephen Duffy from Queen Mary University of London said: “These results demonstrate the inequalities in cancer screening participation, with the lowest levels of participation in the areas of highest deprivation.
“Since most women had at least one form of screening, we know that there isn’t an objection to screening as a whole. However, individuals find some screening procedures less acceptable than others, so the key to improving participation is making the screening experience better.
“We’ve seen this work with a new and less burdensome test in bowel cancer screening, which was considerably more acceptable and resulted in a substantial increase in uptake. Most encouragingly, the greatest improvements in uptake were seen in those who previously had the lowest participation levels.”
Systemic lupus erythematosus (SLE) is a multisystemic autoimmune disease, the spectrum of which covers a wide array of clinical and laboratory manifestations. The disease has considerable clinical and immunological heterogeneity; no two patients with SLE are exactly alike. The aetiology of SLE is thought to be multifactorial, with multiple genetic, epigenetic, hormonal, and immunopathological pathways being involved. SLE is characterised by the production of autoantibodies which leads to immune complex deposition, inflammation, and eventually, permanent organ damage. The course of SLE is largely unpredictable and characterised by periods of remission and disease exacerbation that could lead to progressive organ damage and dysfunction. It most commonly presents in women with a peak incidence between the ages of 15 and 40.1 However, SLE can affect all age groups, from infants to geriatric patients. Compared with the age- and sex-matched general population, SLE is associated with at least a five-fold increase in mortality.2
Accurate clinical assessment of the disease is desirable because SLE has a complex phenotype, a cumulative damage and a variable disease course with new organ system involvement even many years after diagnosis.
For all these reasons, the availability of measures for diagnosing, monitoring disease activity, and assessing tissue damage are all important and necessary in SLE management. Assessment of SLE3 can be divided into several components:
All patients suspected of having SLE should be referred to a rheumatologist or to a SLE specialist to confirm diagnosis and be involved in ongoing care. When considering a patient with a possible diagnosis of SLE, a detailed clinical history and examination is required in order to identify relevant clinical features. An early diagnosis of SLE could be challenging for many reasons: the absence of pathognomonic findings, the heterogeneity at onset and the time required for its full development. This peculiar disease pattern could explain the long delay reported between the onset of the first symptoms and the final diagnosis. In the last decades, however, the delay between clinical onset and diagnosis has been reduced, from more than 2 years in patients diagnosed during the eighties to nine months in those diagnosed in the last years.4 The Systemic Lupus International Collaborating Clinics (SLICC) set of classification criteria (Table 1)5 and the new American College of Rheumatology and European League Against Rheumatism (ACR/EULAR ) criteria (Table 2)6 may be helpful also considering the diagnosis; however, they do not cover all the possible clinical manifestations of SLE.
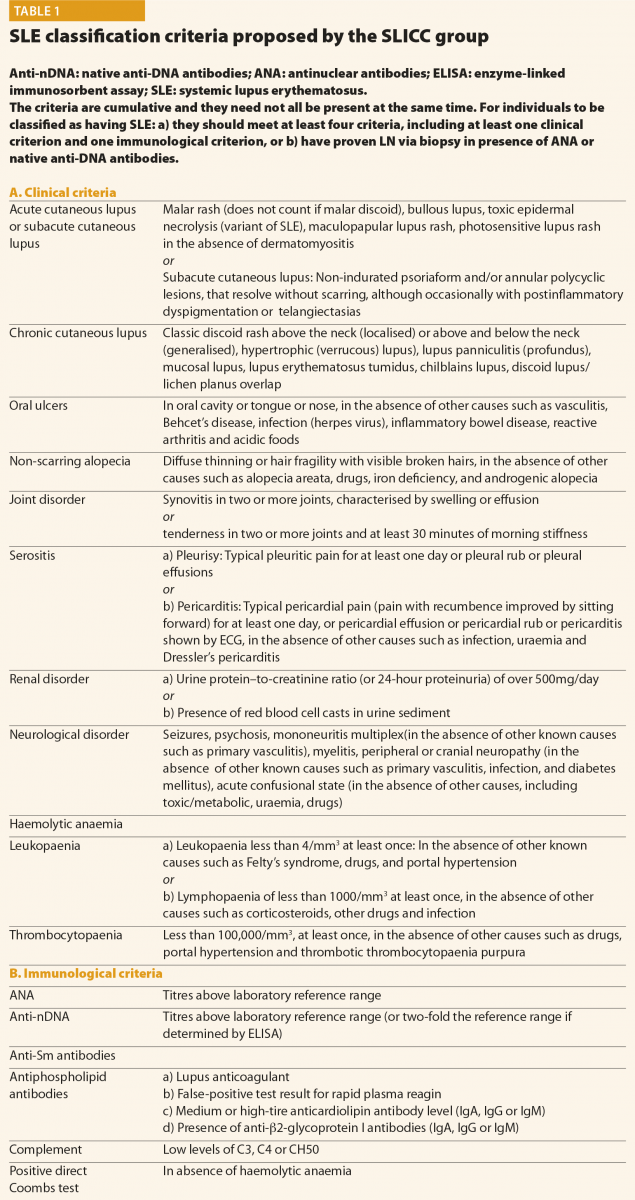
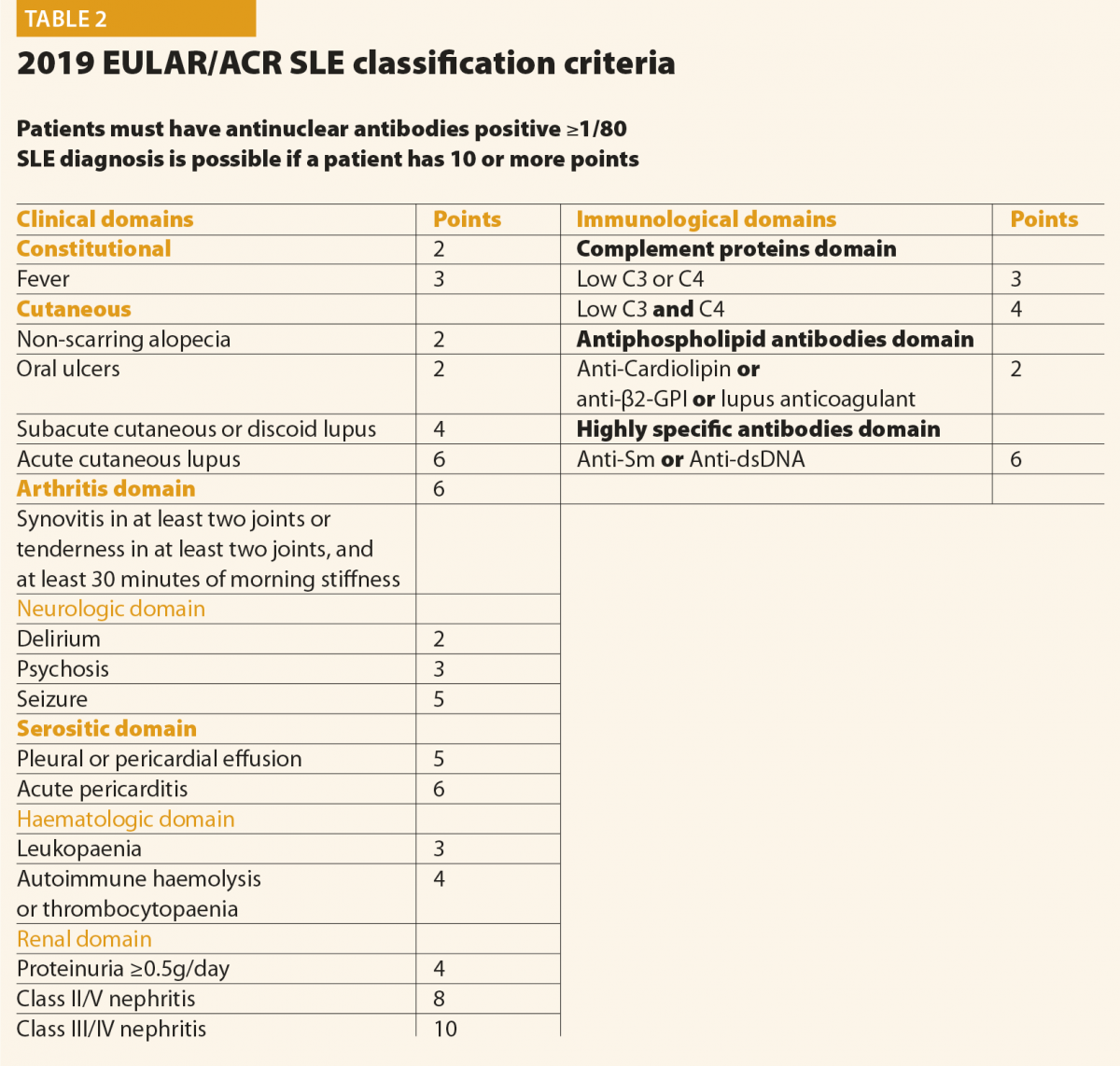
In conclusion, final diagnosis of SLE is a combination of clinical features and the presence of at least one relevant immunological abnormality and it still requires the meticulous clinical judgment of qualified physicians.
Screening questions should be employed to detect possible SLE manifestations in all systems of the body, particularly manifestations included in the classification criteria and others that are common in lupus patients, for example, fatigue, photosensitivity, skin lesions, arthralgias, alopecia and Raynaud’s phenomenon. Constitutional symptoms such as fatigue, fever, unintentional weight loss and lymphoadenopathy are common presenting symptoms of SLE. These symptoms, however, are not specific and other causes should be excluded, such as infection, malignancy, endocrinopathy, other connective tissue diseases, depression and fibromyalgia. In addition, environmental triggers such as exposure to ultraviolet radiation, infection, or the use of certain medications, should be identified, if possible. At the moment of the diagnosis, a complete clinical and immunological evaluation should be performed, as reported in Table 3. Possible kidney or neurological involvement should always be ruled out, because involvement of these organs is considered as a severe SLE manifestation.
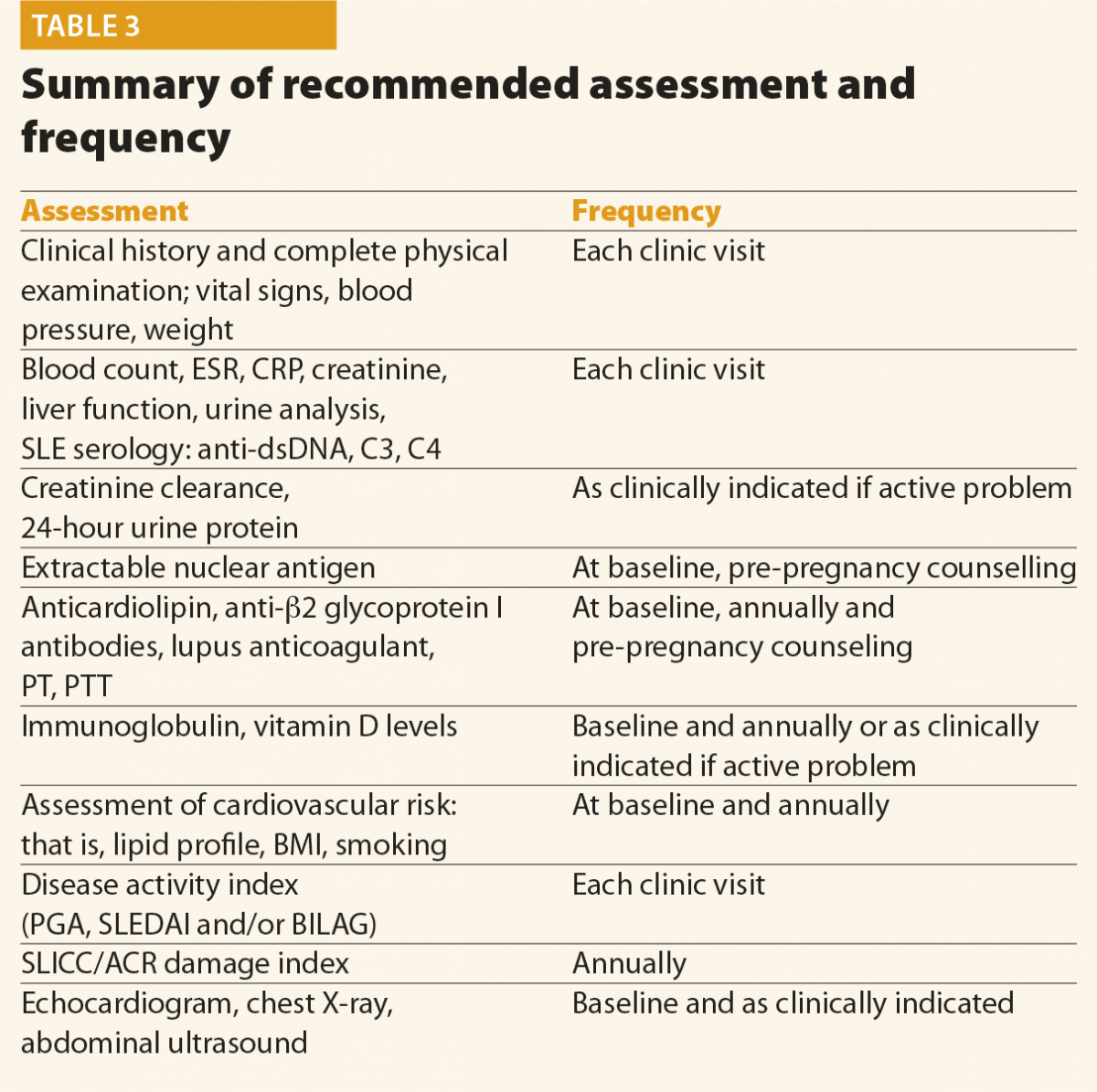
No data are available in the literature to suggest an optimal frequency of clinical and laboratory assessment in patients with SLE. Table 3 summarises the assessment and monitoring of patients with SLE.3
The frequency of the follow-up visits should be based on the activity and severity of the disease, its complications and evolution. In patients with inactive disease, without organ damage and comorbidities, the EULAR recommends that clinical and laboratory examinations should be carried out every 6–12 months.7 Patients with active or more severe disease, with complications related to the treatment, as well as when immunosuppressant treatment is introduced or reduced, will need more frequent control.
How to monitor a SLE patient
SLE is a very heterogeneous disease and its activity fluctuates over time. This variability means that patients with SLE require standardised and objective monitoring of the disease, with validated instruments to determine the degree of activity.
The use of an activity index is also desirable in routine clinical practice as a way of guiding therapeutic decisions as objectively as possible. Moreover, clinicians have to be able to distinguish disease activity from chronic damage, infection and other comorbid disease, including drug side effects.
Many indices have been proposed and all of these have been proven to be valid to measure the activity of SLE and they are also able to predict damage and mortality.8 In addition to the Physicians’ Global Assessment (an estimate of activity rated on a 0 to 3 visual analogue scale), the most common measures used include the SLE Disease Activity Index (SLEDAI), the British Isles Lupus Assessment Group (BILAG), the Systemic Lupus Activity Measure (SLAM), the Lupus Activity Index (LAI), and the European Consensus Lupus Activity Measurement (ECLAM).
The SLEDAI index and its latest version SLEDAI-2K9 is one of the most commonly used global disease activity measure in clinical practice and clinical trials (Table 4). The score has demonstrated validity, reliability, and sensitivity to change in several observational studies. The practical applicability of SLEDAI-2K in clinical settings, its ease of administration (less than 10 minutes to completed), and its simplicity in scoring are fundamental properties. The SLEDAI-2K includes the evaluation of 24 weighted objective variables (16 clinical and 8 laboratory; Table 4). A manifestation is recorded if it is present over the past 10 days. The sum of the items, identified in a single patient, corresponds to the disease activity. Concerning the laboratory items, the SLEDAI includes the determination of complement levels and of anti-dsDNA antibodies. The main limitation is that the SLEDAI-2K is not able to capture improvements or worsenings within an organ system, and it does not include severity. By contrast, the BILAG score10 (Figure 1) refers to disease activity in nine organ systems
in the previous 30 days. For each item included, a score is assigned according to the degree of disease activity. The BILAG is the most comprehensive activity index, and it has the power to detect changes in the activity of the disease, therefore it is frequently used in randomised clinical trials. However, it is longer than SLEDAI to complete (almost one hour), it requires several laboratory parameters and
a dedicated software. In conclusion, it is not feasible in everyday practice.
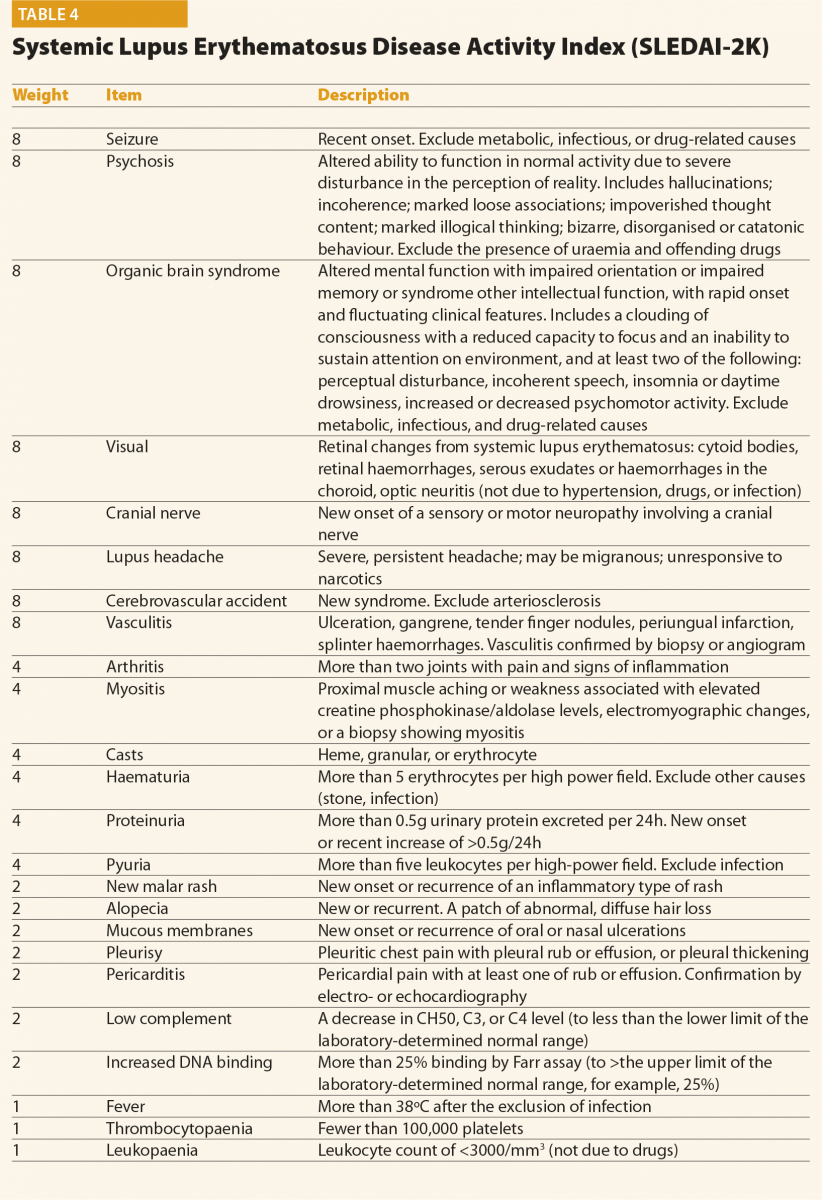

Other than disease activity, damage caused by the disease also has to be assessed. Since 1996, a damage index proposed by Systemic Lupus International Collaborating Clinics has been used – the SLICC/ACR Damage Index (SLICC/ACR DI).11 This index includes persistent damage related to SLE per se (present for more than six months), specific comorbidities associated to the autoimmune disease, and features that are often due to toxicity related to treatments. Higher damage index scores early in disease have been associated with a poor prognosis and with increased mortality.12 Thus, the SLICC/ACR DI complements other measures of disease activity described above, and it is an important outcome measure. It is usually completed (or updated) yearly.
Lifestyle guidance
Finally, lifestyle modifications should be discussed. All SLE patients should stop smoking, not just because of the effect that smoking has
on the activity of the disease and quality of life,13 but also because of its causal association with the increase in risk of cardiovascular disease, infection and cancer. Regular physical exercise in people with stable SLE with low to moderate disease activity should be recommended in order to avoid weight gain expecially in those patients that are taking corticosteroids.
Adequate photoprotection should be advised in all SLE patients, irrespective of the presence of skin manifestations.14 Broad spectrum photoprotectors with high solar photoprotection index should be applied in adequate quantity before exposure and frequently reapplied and
the use of hat and long-sleeved clothing should be advised.
Patients with SLE are at increased risk of comorbidities such as infection, thrombotic events, premature cardiovascular and peripheral vascular disease and osteoporosis. These aspects should be assessed at diagnosis and regularly during follow-up. The frequency of the proposed assessment is reported in Table 3.
Antiphospholipid antibodies, thrombosis and cardiovascular risk
Antiphospholipid antibodies (aPL) are considered a risk factor of thrombosis, both in the presence and in the absence of a concomitant autoimmune disease such as SLE.15
It is very important to remember that all the three test, namely lupus anticoagulant (LA), anticardiolipin (aCL) and anti-β2glycoprotein
I antibodies (anti-β2-GPI) should be determined at baseline and regularly during the follow-up because only their complete evaluation allow to assess the risk profile. It has been demonstrated that the combinations of aPL generally increase the risk of thrombosis, and the triple positivity is associated with the greatest likelihood of thromboembolic events.16
The decision for the use of primary prophylaxis in aPL carriers remains challenging: patients at ‘high risk’ (that is, triple positivity, high titres of autoantibodies) should be treated with low-dose aspirin, whereas in patients considered at ‘low risk’, the decision should be made taking into consideration also the classical cardiovascular risk factors, age and the risk of bleeding.
Concerning the secondary prevention, for both unprovoked venous thromboses and arterial thrombosis, indefinite anticoagulation is recommended.17
The use of novel oral anticoagulants for secondary prevention should be avoided, especially in patients with triple aPL positivity.18
It is well known that SLE patients present an increase in risk of cardiac events19 and coronary disease compared with the rest of the population. The risk that a young female SLE patient develops cardiovascular events was reported 30–50-times higher than that of the same age healthy control. This excess of risk is not entirely explained by classical risk factors, but rather, other factors related to the actual disease may also be involved, such as chronic systemic inflammation or treatment with glucocorticoids.19,20
Traditional risk algorithms (for example, Framingham) show only marginal differences between SLE and controls, and thus underestimate cardiovascular disease risk of SLE patients. A new risk score to predict the development of cardiovascular disease has been reported (QRISK3)21 where SLE is considered as a risk factor. The use of QRISK3 was able to capture significantly more patients with SLE
with an elevated 10-year risk of developing cardiovascular disease compared with Framingham.22 The European Soiety for Cardiology includes SLE in the population group with increased risk of suffering cardiovascular disease,23 for whom it recommends a target LDL level lower than 2.5mmol/l (96mg/dl).
Infection
Patients with SLE are at high risk for infections because of the underlying immune aberrations and the prolonged use of immunosuppressive treatments.24,25 Protection against infections should focus both on primary prevention, as well as promp recognition and treatment.
Patients with SLE should receive vaccinations as proposed by EULAR in the recommendations for vaccination of patients with autoimmune rheumatic diseases.24 Seasonal influenza and pneumococcal vaccination are strongly recommended for patients with SLE, preferably during stable disease.24,26 Live attenuated vaccines are not recommended in patients chronically treated with immunosuppressant therapies because of the risk of disseminated infections.
Osteoporosis
Osteopaenia and osteoporosis are frequent comorbidities in SLE patients and the disease itself represents an independent risk factor for low bone mineral density (BMD), but there are additional risk factors that may concur, such as therapy with glucocorticoids and the high prevalence of vitamin D insufficiency or deficiency,27 favoured also by the doctor’s prescribed lifestyle. BMD score should be evaluated at disease onset, and the ongoing EULAR recommendations suggest the supplementation of elemental calcium and cholecalciferol in all patients chronically treated with low-medium dosage of corticosteroids.28
In case of vitamin D deficiency, higher dosage should be initially prescribed and then lowered to a standard dosage of cholecalciferol (600–800IU/day). In patients with SLE aged >40 years, who have a moderate to high risk of a major osteoporotic (>10%) or hip fracture (>1%) within 10 years (as assessed by the fracture risk assessment tool), antiresorptive therapy is recommended if there are no contraindications.
Clinicians involved in the care of SLE patients should frequently discuss the topic of family planning with all patients of childbearing age. Family planning and contraception should be a topic of discussion from the early phases of the disease.
In the past, SLE was considered to be an absolute contraindication to pregnancy, but maternal and foetal outcomes in these women have greatly improved thanks to a correct timing of pregnancy, close monitoring, multidisciplinary management and an increased knowledge about the medications that can be used during pregnancy and breastfeeding.29,30
Ideally, all patients with SLE who wish to conceive should have preconception counselling; in fact, planned pregnancies have demonstrated better maternal and foetal outcomes. Systemic activity of SLE must be assessed, with careful attention to renal involvement. A recent flare is a risk factor for recurrence during pregnancy; therefore a pregnancy should be planned after
at least six consecutive months of stable disease. During the preconceptional visit, the SLE specialist should:
At the same time, patients need to be evaluated by an experienced obstetrician who will perform the investigation required for all the women who wish to get pregnant.
Once pregnancy is confirmed, monthly visits are usually indicated (or even more frequently)
if the disease is not controlled.
Special monitoring is dedicated to women with Ro/SSA and/or La/SSB antibody positivity; these patients should be informed about the risk of neonatal lupus and congenital heart block, a severe complication that occurs in 0.7–2%31,32 of women with these autoantibodies.
Physicians involved in the care of patients affected by SLE need easy to use, replicable and practical evaluation tools. These can help in all disease phases, from diagnosis through to the occurrence of flares, comorbidities, or even pregnancy. SLE shows a variable and complex phenotype; therefore a precise assessment is needed to administer the treatment able to target the index manifestation. Only by following this strategy will we be able to control the disease, reaching remission or low disease activity,26 which are essential in improving
the morbidity, mortality and quality of life in these patients.
References
1 Wahren-Herlenius M et al. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet 2013;382:819–31.
2 Mok CC et al. Life expectancy, standardized mortality ratios, and causes of death in six rheumatic diseases in Hong Kong, China. Arthritis Rheum 2011;63:1182–9.
3 Mosca M et al. European League Against Rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann Rheum Dis 2010;69:1269–74.
4 Doria A et al. SLE diagnosis and treatment: when early is early. Autoimmun Rev 2010;10:55–60.
5 Petri M, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86.
6 Aringer M et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus.Ann Rheum Dis 2019;78(9):1151–9.
7 Mosca M et al. Development of quality indicators to evaluate the monitoring of SLE patients in routine clinical practice. Autoimmun Rev 2011;10:383–8.
8 Griffiths B et al. Assessment of patients with systemic lupus erythematosus and the use of lupus disease activity indices. Best Pract Res Clin Rheumatol 2005; 19:685–708.
9 Glad-man DD et al. Systemic Lupus Erythematosus Disease Activity Index 2000. J Rheumatol 2002;29:288–91.
10 Hay EM et al. The BILAG index: a reliable and valid instrument for measuring clinical disease activity in SLE. Q J Med 1993;86:447–58.
11 Gladmand D et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–9.
12 Rahman P et al. Early damage as measured by the SLICC/ACR damage index is a predictor of mortality in systemic lupus erythematosus. Lupus 2001;10(2):93–6.
13 Ghaussy NO et al. Cigarette smoking and disease activity in systemic lupus erythematosus. J Rheumatol 2003;30:1215–21.
14 Vilá L et al. Association of sunlight exposure and photoprotection measures with clinical outcome in systemic lupus erythematosus. P R Health Sci J 1999;18:89–94.
15 Miyakis S, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4: 295–306.
16 Chighizola CB et al. The treatment of anti-phospholipid syndrome: a comprehensive clinical approach. J Autoimmun 2018;90:1–27.
17 Tektonidou MG et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis 2019; Epub ahead of print doi:10.1136/annrheumdis-2019-215213.
18 Pengo V et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018;132:1365–71.
19 Magder LS, Petri M. Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am J Epidemiol 2012; 176: 708-19.
20 Esdaile JM et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum 2001;44:2331–7.
21 Hippisley-Cox J et al. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ 2017;357:j2099
22 Edwards N et al. QRISK3 improves detection of cardiovascular disease risk in patients with systemic lupus erythematosus. Lupus Sci Med 2018;5:e000272.
23 Perk J et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Atherosclerosis 2012;223:1–68.
24 Elkayam O et al. Update of EULAR recommendations for vaccination of patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2018;77.
25 Rúa-Figueroa I et al. Incidence, associated factors and clinical impact of severe infections in a large, multicentric cohort of patients with systemic lupus erythematosus. Semin Arthritis Rheum 2017;47:38–45.
26 Fanouriakis A et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78(6):736–45.
27 Almehed K et al. Prevalence and risk factors of osteoporosis in female SLE patients-extended report. Rheumatology (Oxford) 2007;46:1185–1190.
28 Grossman JM et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2010;62:1515–26.
29 Andreoli L et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis 2017;76(3):476–85.
30 Götestam Skorpen C et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 2016;75(5):
795–810.
31 Brucato A et al. Risk of congenital complete heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum 2001;44:1832–5.
32 Fredi M et al. First report of the Italian Registry on immune-mediated congenital heart block (Lu.Ne registry). Frontiers Immunology 2019;6:11.
16th September 2019
Many hospitals with active oncology departments require access to pathology laboratories to enable a complete molecular profile of a tumour from relevant biopsies. At the same time, new drugs for targeted therapy and immunotherapy are becoming available and many of them are used in combination or sequentially to avoid resistance mechanisms. However, a consequence of this revolution in cancer treatment is that a new challenge is looming on the horizon for the pathologists: that is, to work beyond the diagnosis and classification and directly contribute to optimising rapid patient treatment with sustainable costs.
The choice of analytical methods depends on clinical requirements, availability of space, expertise of the staff and dedicated budget. In our Unit, we chose a multimarker-approach based on next generation sequencing (NGS) platforms (for example, ThermoFisher Scientific). Serial testing takes time and depletes tumour tissue and the cost of single-gene methods scales linearly with the number of interrogated genes, which led us to choose our current approach. The ASCO endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update1 stated that multiplexed panels are likely to be more efficient in terms of cost and tissue requirements than other technologies.
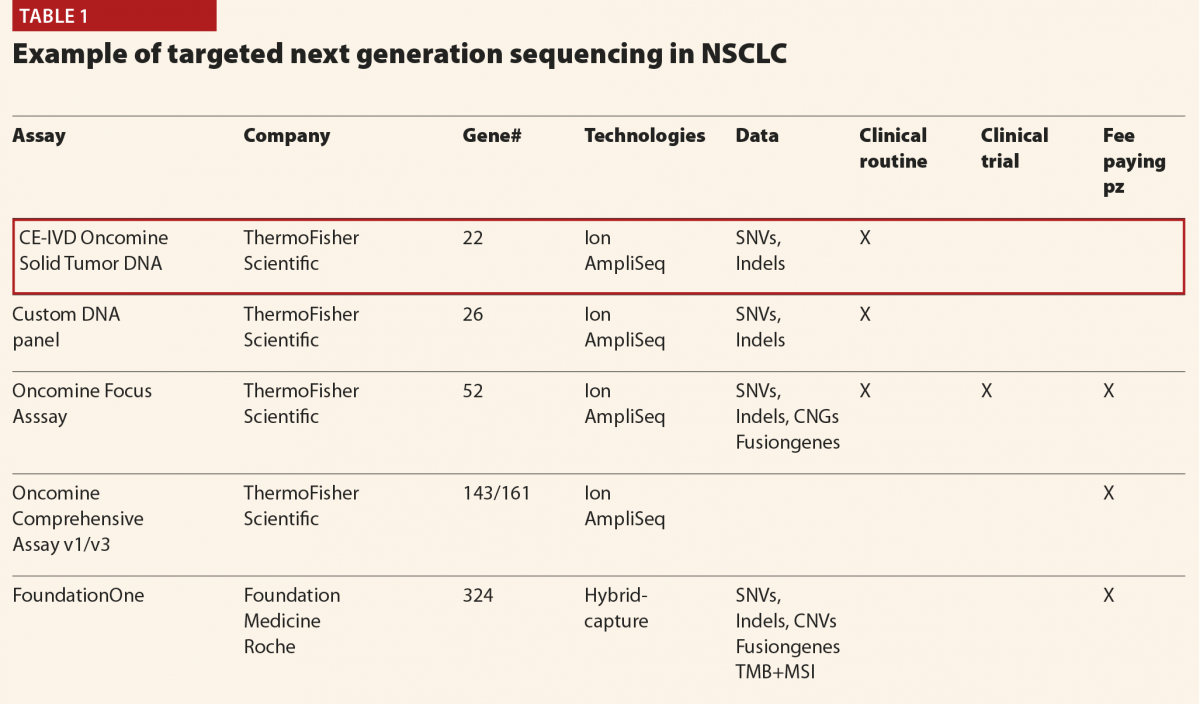
Currently we receive 4000 requests of molecular tests per year mainly for advanced solid tumours (non-small cell lung cancer (NSCLC), colorectal carcinomas and malignant melanomas represent 80% of the samples). A panel of 22 genes (Oncomine Dx Solid Tumors) is offered to all the patients, whereas more complex panels (Oncomine Focus and Oncomine Comprehensive Assays) identifying not only mutations but also copy number variations, and fusion genes can be used in selected cases
(Table 1).
Immunotherapy is transforming oncology and its use in many solid tumours and Hodgkin’s lymphoma has become a stronghold in the changing landscape of cancer treatment. Immune checkpoint inhibition is exemplified by the introduction of anti-PD-1 or anti-PD-L1 antibodies. The evaluation of PD-L1 expression in tumour cell sand/or inflammatory cells is the most widely used predictive assay. However PD-L1 has a heterogeneous expression pattern and different antibodies are available for this assessment. There are different evaluation schemes in different tumours to assess PD-L1 positivity and match the patient to the therapy. Moreover, it is well known that some patients having tumours with high PD-L1 expression might show hyper-progression during anti-PD1–PD-L1 therapy. To overcome these issues, a potential biomarker has been introduced recently: namely, the tumour mutation burden (TMB).
Patient-specific neoantigens that develop as a result of somatic mutations can induce a T-cell response. A high number of mutations does not always result in neoantigens, but it does increase the probability of developing neoantigens. This observation is promising as studies have shown that mutational landscape determines sensitivity to PD-1 blockade in NSCLC and that PD1 inhibitors in patients with a higher non-synonymous TMB in their tumours have a greater efficacy. We now know that it is possible to use wide NGS panels instead of whole exome sequencing to calculate TMB, but there are great differences in NGS gene panel content, variant filtering methods and definitions of high TMB thresholds. So it is difficult to compare TMB results across studies. In clinical practice, we need an ‘all in one assay’ to give a complete answer to the patient and to the oncologist. For a complete evaluation of the genetic aberrations present in the tumour sample (mutations, copy number alterations, fusion genes, TMB and MSI) there are only two validated and FDA approved NGS panels: Foundation One Dx and MSKCC Impact. However, the epidemiology of NSCLC makes it impossible to use these assays in all the cases. Other panels are currently available (ThermoFisher, for example) or soon to be available (Illumina panel with 500 genes). In Europe, we are not obliged to work with companion diagnostics, but with validated assays, therefore it is plausible that different panels could be used. In this sense a harmonisation process should be welcome. Reliable assays at a sustainable cost could offer a solution.
In immunotherapy, as in targeted therapy, the era of ‘one size fits all’ is past and the day-to-day work of pathologists is changing dramatically.
More and more information must be given in the same time and NGS technology is currently the only approach for this goal. In advanced solid tumours diagnosed on small biopsies and/or cytological samples, the real issue is the quantity of the specimen.
Today more than ever is ‘the tissue the issue’.
Reference
1 Kalemkerian G et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J Clin Oncol 2018;36:911–19.
In July 2018, the National Institute for Health and Care Excellence (NICE) published revised guidelines for the management of rheumatoid arthritis (RA) disease in adults.1 Some clinicians will find it challenging to adhere to these, but they reflect best practice. Management of rheumatoid arthritis depends on a multidisciplinary approach and shared care between secondary and primary care. The guideline is relevant to non-specialist health professionals who are involved in the initial assessment of RA symptoms and ongoing care of people diagnosed with RA. What are the implications of these guidelines for commissioners and providers of services for people with RA?
RA is a chronic, disabling autoimmune disease characterised by synovitis of small and large joints causing swelling, stiffness, pain, and progressive joint destruction. Approximately 1% of the UK population have RA, and as many as 15% of these people may have severe active disease at any point in time. It affects roughly three times as many women as men. People tend to develop RA between 40 and 60 years of age, although it can occur at any age. The early signs of rheumatoid arthritis of joint pain and swelling usually present in primary care. Fast and accurate referral to rheumatology services is important to achieve early remission and prevent or reduce disability.2
The management of RA has evolved in the nine years since the previous NICE guideline on RA was published, with greater emphasis on a treat-to-target strategy rather than specific drug regimens,3 and debate about the merit of initiating treatment with combination drug therapy.4 Technologies such as ultrasound have been increasingly used for diagnosis and monitoring of synovitis where it is unclear from clinical examination.5 These aspects of management were investigated by the Guideline Committee, and recommendations have been updated using new evidence, leading to changes to the recommendations for treatment with conventional synthetic disease modifying anti-rheumatic drugs (csDMARDs), glucocorticoids for bridging treatment, and choice of treatment for symptom control. Several aspects of the guideline have remained unchanged since its publication in 2009.
NICE publishes evidence-based recommendations for health and care in England (not Wales or Scotland, although they can also be used there). The express aim of the Institute is to prevent ill health, to promote and protect good health, to improve the quality of care and services and to adapt and provide health and social care services. The guidelines are widely used to define ‘minimum standards of care’ in the UK, so that patients and carers using the National Health Service (NHS) know what they are entitled to receive from healthcare providers. Commissioners and Trusts are expected to adhere to NICE guidelines and to assure the process through regular audit. If this does not happen, then providers would be open to censure, for example by the Health Service Ombudsman in the event of a complaint, and may lose their eligibility to bid for provision of specialised services.
NICE also publishes quality standards in the form of statements that are designed for commissioners and providers to identify gaps in service provision and areas for improvement, to facilitate measurement of quality of care and demonstration of high quality care, with the aim to facilitate commissioning of high quality services. The Quality standards for RA were last published in 2013 but are currently being revised. Clinicians would normally be expected to undertake regular audit against these standards, and commissioners might be expected to receive assurance that this is undertaken.
The new recommendations are:
Referral
The guideline emphasises the importance of rapid referral to a rheumatologist for any adult with suspected persistent synovitis of undetermined cause independent of investigations including blood tests for acute phase response or rheumatoid factor. The guideline recommends referral in any patient when:
Referral should be guided by clinical examination and should not be delayed by waiting for results of any investigations as they may be normal especially in early disease. When positive, anti-cyclic citrullinated peptide (CCP) antibodies and/or radiographic erosions at diagnosis in combination with a raised C-reactive protein (CRP) are indicators of a poor prognosis.
It is recommended that CCP, CRP and X-rays are arranged at initial diagnosis in secondary care if they were not undertaken before referral.
The guideline recommends that the rheumatologist should inform those with risk factors of a poor prognosis that they have an increased risk of radiological progression. This is in order to emphasise the importance of the patient monitoring their condition and seeking rapid access to specialist care if disease worsens or they have a flare.
Treat-to-target strategy
Disease activity can be measured by various tools, such as the DAS28, which is based on a composite score from clinical assessment of the number of tender joints, swollen joints, global pain, and a biomarker for inflammation (either erythrocyte sedimentation rate (ESR) or CRP). Definitions of remission or low disease activity vary according to the measure used. For example, with the DAS28, remission is a score of <2.6 and low disease activity is ≤3.2. The previous guideline did not recommend a specific target other than agreeing a target with the patient. The revised guideline now recommends that patients with active RA are treated with the aim of achieving a target of remission or low disease activity if remission cannot be achieved (treat-to-target). This is more challenging than the recommendation in 2009 and could have resource implications as patients might have more treatment and follow up appointments. The guideline also recommended that clinicians should consider making the target remission rather than low disease activity for people with an increased risk of radiological progression (that is, those with positive anti-CCP antibodies or erosions on X-ray at baseline assessment).
Pharmacological management
Initial pharmacological management is led by specialists. Conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) are differentiated from targeted synthetic (ts)DMARDs (such as Janus kinase inhibitors) and biologic (b)DMARDs (including inhibitors of cytokines such as tumour necrosis factor and interleukin-6), which were not within the scope of this guideline. The previous guideline recommended initial treatment with a combination of two or more csDMARDs including methotrexate. However, the evidence for this approach was re-evaluated and a meta-analysis did not find superiority for any individual drug. This is surprising to those who consider methotrexate to be superior although this is not supported by current data. Extensive literature review also did not find superiority for initial combination compared with a step up strategy. In contrast to the previous recommendation, the current guideline therefore recommends initiation with a single csDMARD (either sulfasalazine, methotrexate, or leflunomide) and sequentially adding further drugs in a step-up approach if the target is not met. Thereafter, if the patient remains with severe active disease (DAS>5.1) they would be eligible for a bDMARD. NICE at present do not have a recommendation for those who have not met the target of at least low disease activity and do not have severe active disease; this is currently under review because of the reduction in the cost of some drugs following the introduction of tsDMARDs and biosimilar bDMARDs.
Once a patient has achieved and maintained their treatment target of remission or low disease activity for at least a year without glucocorticoids, the guideline recommends the rheumatologist should consider cautiously reducing drug doses or stopping drugs in a step-down strategy but to return promptly to the previous DMARD regimen if the treatment target is no longer met.
Frequency of follow up
What has proved challenging for some rheumatologists was the recommendation in the 2009 guideline to follow patients monthly until their target was met. However, although this may have resource implications the recommendation remains that all patients should be reviewed monthly in their rheumatology unit until they are in remission or low disease state. Thereafter it is recommended that patients should have a review appointment after six months to ensure that the target has been maintained and if stable to be reviewed at least on an annual basis. The annual review should be a comprehensive evaluation and include an assessment of disease activity and damage and any need for surgery, a measure of functional ability (using, for example, the Health Assessment Questionnaire) and impact on life, a check for the development of comorbidities, such as hypertension, ischaemic heart disease, osteoporosis and depression, an assessment of symptoms that suggest complications such as vasculitis and disease of the cervical spine, lung or eyes, and appropriate cross-referral within the multidisciplinary team. An annual review was also included in the previous guideline but many rheumatologists have found a comprehensive review to be difficult to deliver. The availability of specialist nurses is often instrumental in supporting these recommendations and service planning should consider the resources required to deliver both monthly monitoring and annual review. Although labour intensive, this approach may prove cost effective by reducing the number of patients who need to be prescribed a bDMARD.
Corticosteroids
One problem with csDMARDS is that they have a gradual onset of action over weeks to months. In order to provide a rapid reduction in symptoms, most rheumatologists recommend short term bridging treatment with glucocorticoids (oral, intramuscular, or intra-articular). The guideline committee were unable to strengthen the recommendation and advise all patients to receive bridging therapy because of the lack of research evidence. However, rheumatologists should be encouraged to prescribe short term steroids when initiating or changing a DMARD and also for disease flares. The guideline recommendation is to “Consider short term bridging treatment with glucocorticoids (oral, intramuscular, or intra-articular) when starting a new conventional synthetic DMARD.”
Symptom control
Although control of synovitis with csDMARD and corticosteroids improves symptoms, some patients require additional analgesia. The committee found very limited evidence for paracetamol, opioids, and tricyclic antidepressants for symptom control in rheumatoid arthritis, so the recommendation for “other analgesics” was removed from the update of this guideline and replaced with a recommendation for NSAIDs alone – to consider oral non-steroidal anti-inflammatory drugs (NSAIDs), including traditional NSAIDs and Cox II selective inhibitors, when control of pain or stiffness is inadequate taking account of potential gastrointestinal, liver, and cardio-renal toxicity, and the person’s risk factors, including age and pregnancy. The lowest effective dose for the shortest possible time of NSAIDs was recommended with co-prescription of a proton pump inhibitor and regular review of risk factors for adverse events.
Monitoring
When patients with RA have met their target, monitoring patients on DMARDs should be shared between primary and secondary care. However, flares of disease are characteristic of many patients with RA and there should be rapid access to specialist care for flares and this is emphasised in the guideline. In addition, although the use of ultrasound has expanded in rheumatology as well as other specialties, the role of ultrasound in the management of RA is unclear.5 Following an extensive literature review the conclusion in the guideline was not to recommend ultrasonography for routine monitoring of disease activity in adults with RA.
Implementation
Some rheumatologists who have not adopted a treat-to-target strategy may need a change in practice. This will require revision of local protocols in order that step-up protocols may be implemented rather than initial combination therapy. The recommendation to especially target patients with poor prognostic markers will need to be included in new protocols. In addition, there may be challenges to health professionals in primary and secondary care when explaining risk factors for progression to some patients. Ultrasound scanning of joints is increasing, and the recommendation not to use ultrasound routinely may need to be reflected in the revision of local protocols.
Although current evidence suggests that all people with rheumatoid arthritis should be offered the same management strategy, it is possible that those identified with a risk of poor prognosis should be treated differently. A high priority research recommendation has been included to answer this question. Research is also needed to identify the best use of corticosteroids in RA, and whether ultrasound can improve management. In addition, In view of the considerable difference in cost between subcutaneous and oral methotrexate, further research needs to be undertaken to determine whether there is greater efficacy of subcutaneous methotrexate compared with oral therapy.
Patients with a DAS28 between 3.2 and 5.1 are often referred to as having moderate disease and at present NICE do not have guidance for this group of patients if they have failed csDMARDs; they are not currently eligible for a bDMARD or tsDAMRD unless they have a DAS28 >5.1. This has proved difficult in managing these patients, but with the reduction in costs of bDMARDs it is hoped that revised health economic analyses will find that it will be cost effective for those with moderate disease to be treated with biosimilar bDMARDs.
References
1 National Institute for Health and Care Excellence. Rheumatoid arthritis in adults: management: NICE guideline (NG100). July 2018. www.nice.org.uk/guidance/ng100 (accessed September 2019).
2 Kyburz D et al; physicians of SCQM-RA. The long-term impact of early treatment of rheumatoid arthritis on radiographic progression: a population-based cohort study. Rheumatology 2011;50(6):1106–10.
3 Cohen MD, Keystone EC. Rational therapy in RA: Issues in implementing a treat-to-target approach in RA. Nat Rev Rheumatol 2013;9:137–8.
4 Sethi MK, O’Dell JR. Combination conventional DMARDs compared to biologicals: what is the evidence? Curr Opin Rheumatol 2015;27:183–8.
5 Lage-Hansen PR et al. The role of ultrasound in diagnosing rheumatoid arthritis, what do we know? An updated review. Rheumatol Int 2017;37:179–87.
13th September 2019
Patient dosimetry audit is a legal requirement in the UK under the Ionising Radiation (Medical Exposure) Regulations (IRMER)1 in order that that diagnostic reference levels (DRLs) can be established and used for X-ray imaging examinations. The principles are also applicable internationally as IRMER is itself based on European legislation and recommendations of the International Commission on Radiological Protection (ICRP). DRLs represent radiation dose levels that would be considered typical for a standard patient. They are a means of monitoring patient doses and are a guide to ‘good and normal practice’,2 allowing consistently high doses to be identified and investigated. Patient dosimetry audit is the analysis of data relating to patient doses to calculate average dose indicator values (usually dose length product (DLP) for CT), check adherence to existing DRLs and set new ones where there is enough data.
Patient dosimetry audit is a well established practice in diagnostic radiology. In the UK, National DRLs (NDRLs) are set by Public Health England (PHE)3–5 and guidance on establishing Local DRLS (LDRLs) has been in place for around 15 years.2 The use of electronic systems such as computed radiological information systems (CRIS) has had a profound impact on the scale and efficiency of this process.6,7 Our local system at RRPPS (the Radiation Protection Services of University Hospitals Birmingham NHS Foundation Trust, UK) is based on CRIS downloads analysed using an in-house python software to give average dose indicators by examination and room.
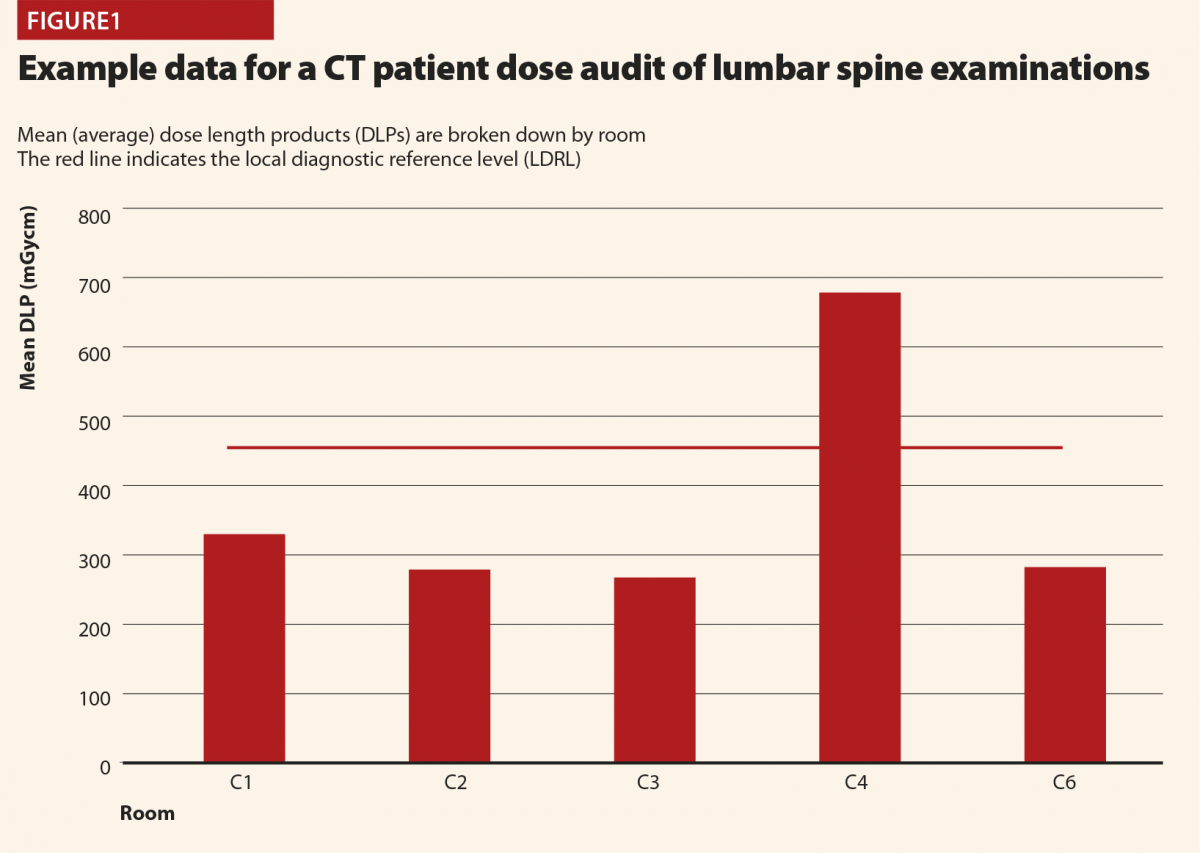
Figure 1 demonstrates how LDRLs are used and established, and gives example data from an audit of CT lumbar spine examinations at a large UK hospital. Mean (average) doses for individual rooms can be compared against an LDRL, which is set as the mean of individual room means. In this case there is clearly a problem with scanner 4, on which the LDRL is consistently exceeded. Having identified that an issue exists via patient dosimetry audit, investigations and corrective action can be implemented. In this case the investigation revealed that whilst all of the scanners were equipped with tube-current modulation, scanner 4 had been set up with a much high reference tube current than the others. This was rectified to harmonise the protocols across all scanners. Additional cases have identified scanners with subtly different imaging protocols and tube-current modulation not being switched on! When there can easily be dozens or more protocols set up on CT scanners, it is usually impractical to check each one line-by-line and compare across every scanner, but simple setup errors will go on to influence doses for many patients. Using patient dosimetry audit to identify such issues and harmonise protocols and practices helps identify and avoid the situation of patients receiving significantly different radiation doses depending on which scanner they happen to find themselves on. In some cases, it might be justified for a particular scanner to have different protocols, and therefore give different doses to others, perhaps because it is used for different clinical conditions (for example, trauma scans). But where there is no such reason, harmonisation of protocols is a simple means of dose optimisation.
CT is also used as standard outside of diagnostic radiology such as in nuclear medicine (with SPECT/CT and PET/CT being routine) and radiotherapy (for treatment planning scans and on-board imaging for verifying patient positioning prior to treatment). Only in the past few years has there been progress in establishing patient dosimetry audit in these areas.8–12 In encouraging recent progress, the Institute of Physics and Engineering in Medicine (IPEM) has established working parties that undertook national audit and published data for nuclear medicine CT13 and radiotherapy planning CT,14 the results of which were subsequently adopted as UK NDRLs.5 This gives very useful data for local results to be compared against. The rest of this discussion will be on the efforts based at RRPPS to establish local systems for patient dosimetry audit in these areas, including some of the challenges we encountered and the solutions developed. In the future, it is hoped similar work will be carried out for radiotherapy on-board imaging as well.
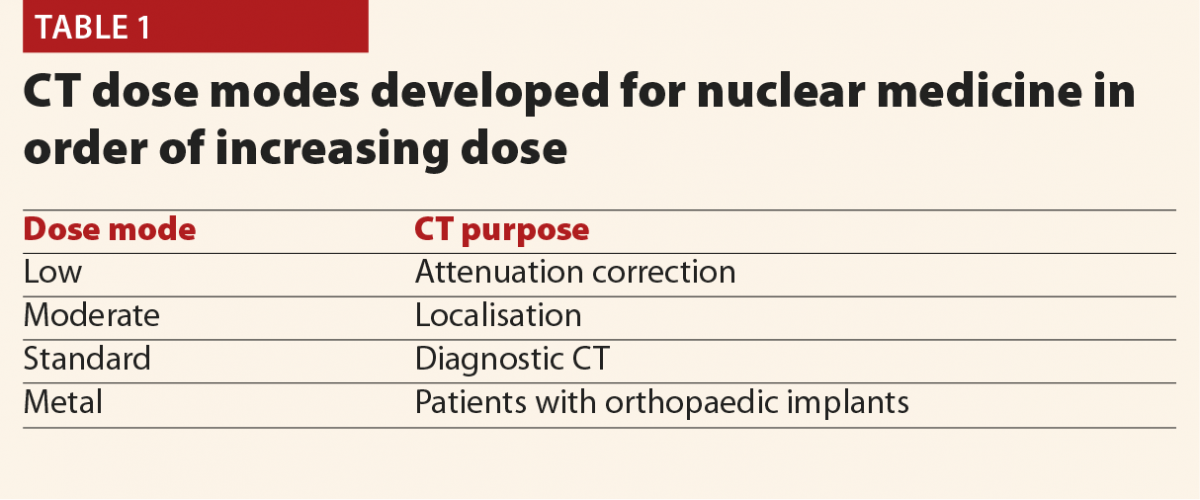
The full results of the initial patient dose audit were published in the British Journal of Radiology15 based on the proposed NDRLs which were available at the time.16 Data were collected for adult patients on two SPECT/CT Scanners (Siemens Symbia T & T16) over November 2014–July 2016 and for a PET/CT scanner (Siemens Biograph mCT Flow) over April–August 2016. Initially, we based the audit on the nuclear medicine examination type (bone scan, parathyroid, etc) as this matches how the NDRLs were set out. However, it soon became apparent that nuclear medicine CT has some complications not found in conventional CT, and so there was more to consider. In discussions between diagnostic radiology and nuclear medicine physicists, two additional considerations were identified; dose mode and body region. Dose mode accounts for the fact that different CT scans had different purposes; for some the CT data was simply used for attenuation correction and so a lower CT dose is needed. Others are full diagnostic quality CT scans, needing higher doses. Table 1 summarises the four dose modes in use in our hospital’s nuclear medicine department. Body region arises due to the use of SPECT-guided CT, in which only the part of the body of interest on the SPECT scan undergoes CT. This is to be encouraged, as it means that the patient’s exposure to X-rays is limited, but does also mean that CT scan lengths vary according to what is scanned and we begin to see descriptions which resemble diagnostic radiology CT; head, t-spine, abdomen and so on as well as the dose mode and examination type. So we must break down the data in order to audit and compare like-for-like. Unfortunately not all of these additional data are captured as standard in CRIS downloads, our standard source of data for patient dosimetry audit. CRIS records captured the nuclear medicine examination type and DLP, so paper records completed after each scan had to be modified to capture dose mode, body region and the scanner used.
Figures 2 and 3 give selected results from this initial audit,15 which highlight some important points. Figure 2 clearly shows that the different dose modes result in significantly different doses, and so they must be considered separately in order to complete a reliable audit. This also highlights that the NDRLs are currently somewhat limited in that they do not fully consider the range of scan purposes, although of course it must be acknowledged that there are limited data currently available and this will hopefully improve in time.
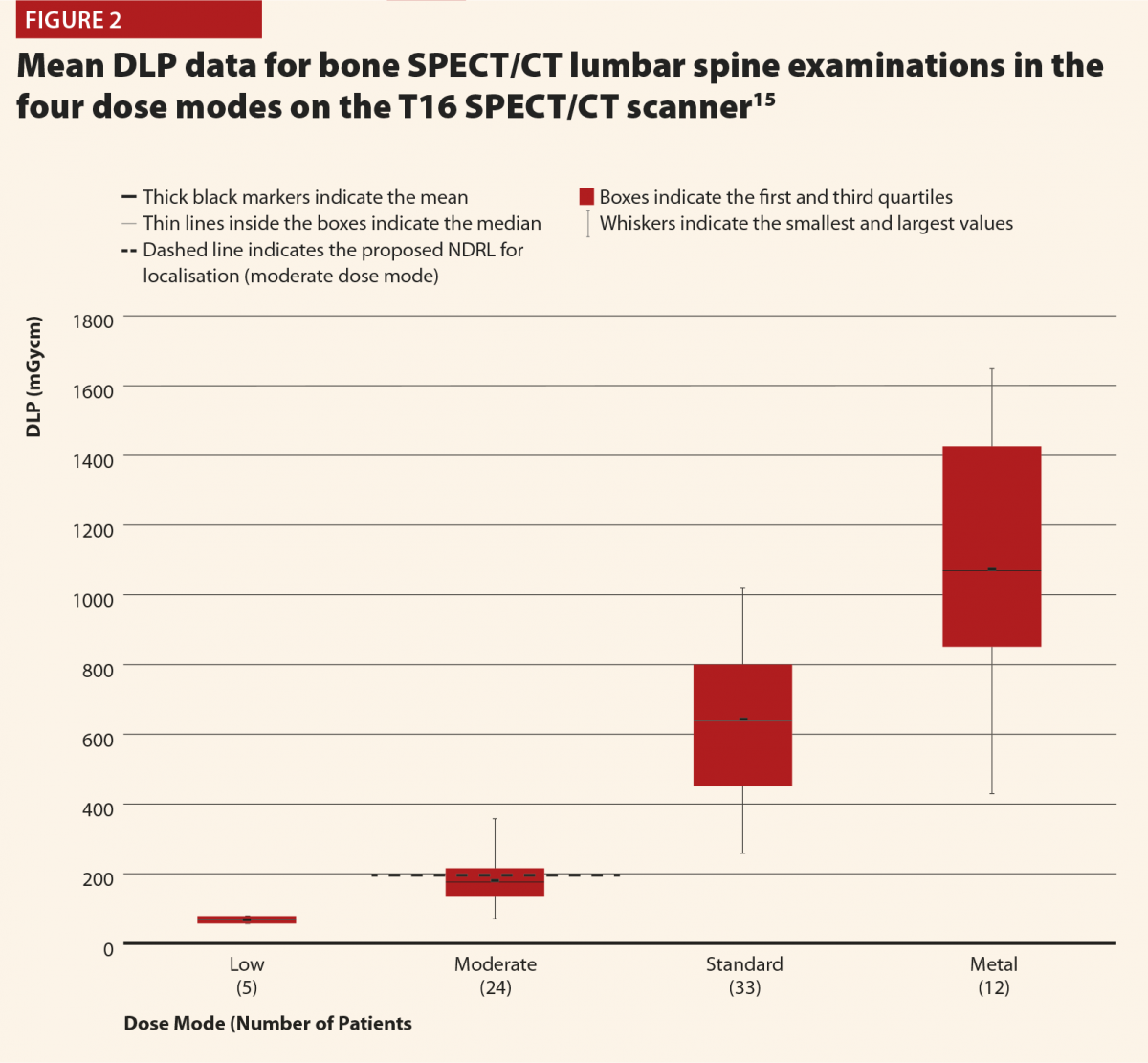
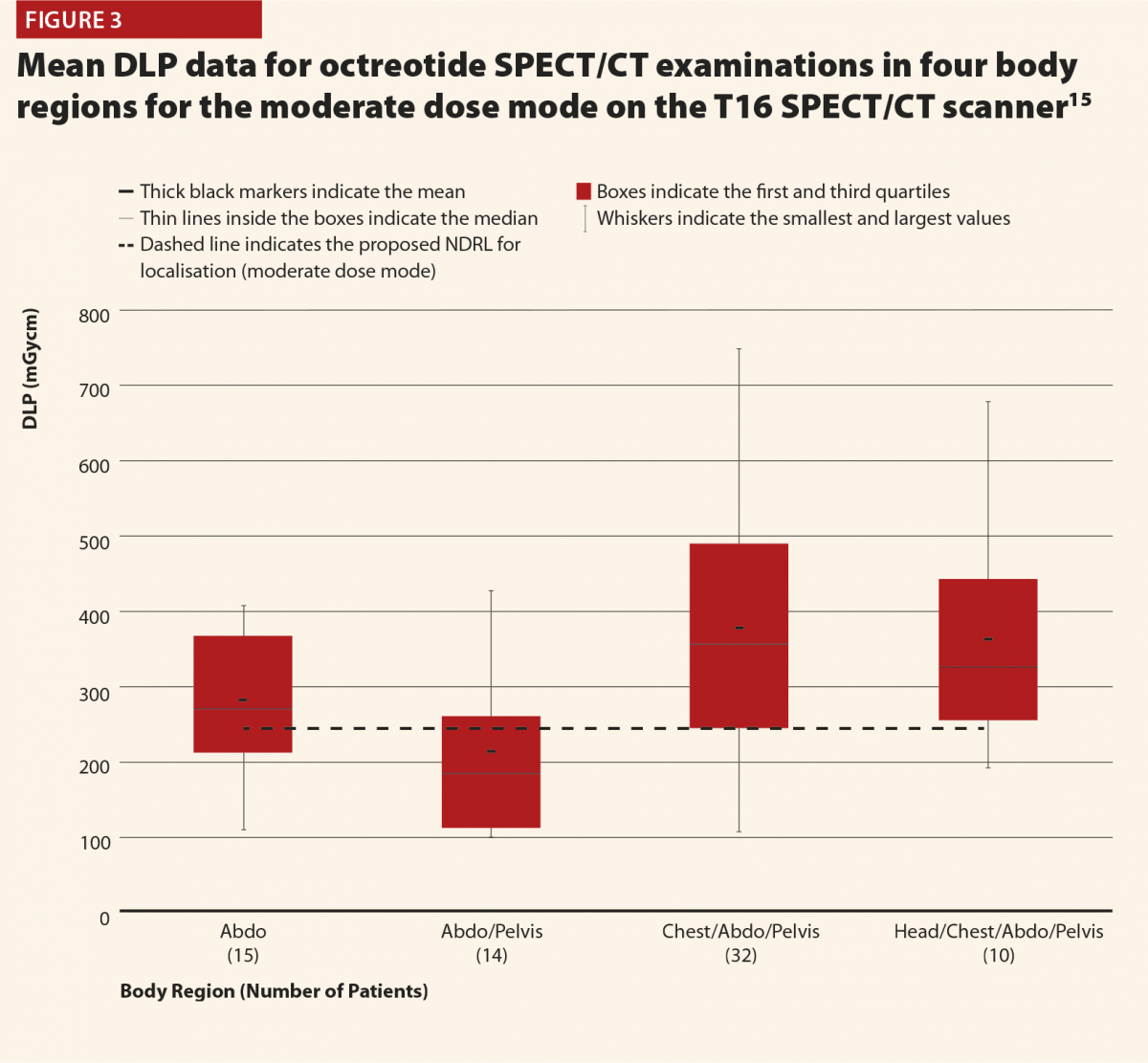
While Figure 3 should be treated with some caution as the numbers of patients are quite low, there is still some useful insight to be gained. There does appear to be a trend that the CT scans covering more than the chest, abdomen and pelvis or more give notably higher doses (and consistently exceed the proposed NDRL). This is to be expected, as longer scans of course give larger DLPs. This raises the question of is there a genuine concern about this exceeded NDRL? This NDRL is set for octreotide examinations, and would therefore represent a collation and average across all body regions scanned. It seems unfair therefore to apply it to longer scans, as they are achieving their clinical aim but will inherently struggle to work within DRLs set based on shorter scans. Further study of the NDRL publication13 reveals that the octreotide data used in this national audit are based primarily on CT scans of the abdomen, meaning that it may not be appropriate to apply the NDRL for longer scans. This highlights the need to consider body regions separately when carrying out the audit.
Overall there are a number of challenges in patient dosimetry audit for nuclear medicine CT. Far fewer nuclear medicine CT scans are carried out compared with radiology CT, and the need to subdivide the data into dose modes and body regions can make it difficult to accumulate sufficient quantity of data for a reliable audit. A solution is to accumulate data over longer periods of time; perhaps two years instead of annually. CRIS did not capture all of the information needed, resulting in a reliance on paper records being transcribed for analysis electronically. This is a very time-consuming process and can result in possible transcription errors. Another potential problem with paper records is inaccurate or inconsistent naming conventions, especially for body regions. For instance, some technologists might write ‘lumbar spine’ and others ‘L-spine’, meaning that any automated analysis will treat these as separate. A significant amount of manual interrogation of data is therefore required to ensure data is recorded correctly and consistently, a very time consuming and inefficient process.
Following on from the initial audit, we have been able to implement improved local systems to facilitate routine audit in a more efficient manner. These include collecting SPECT/CT over a two-year audit period to ensure enough data are included to be confident in it. Additional CRIS entries have been found to record dose mode, and a free text box is used to record body region, removing the need for paper records. Principal technologists also developed guidance on naming conventions to help achieve consistency and reduce the burden of manually correcting entries retrospectively. Finally, a monthly CRIS download is performed and reviewed by a nuclear medicine assistant practitioner to check adherence to naming conventions, confirm the scanner used and body region, and check any ambiguous or suspicious data. These monthly checks are essentially a tidy and quality check of the data, with monthly numbers being found to be more manageable. Therefore the system is not fully automated, but much less labour intensive than previously and with practices improving over time, routine audit can be made sustainable in the long term.
In summary, it is certainly possible to develop systems for patient dosimetry audit in nuclear medicine CT, but auditors must be aware of the additional complications that might not be familiar to those primarily working in diagnostic radiology. Careful thought is needed on how these complications will be managed in an efficient way to allow for ongoing audit.
For radiotherapy planning CT, data were analysed for adult patients on two Philips Brilliance Big Bore CT scanners over the 2017 calendar year. The task was found to be very similar to that for diagnostic radiology CT, in that there were no separate dose modes and the protocols were standardised in terms of the body region scanned. Once the data were acquired, it was therefore simple to analyse using established methods, but there were challenges with the practicalities of acquiring the data. Radiotherapy systems are not interfaced with CRIS, as the scans are not taken to be reported on but to facilitate treatment. Instead, radiotherapy systems include treatment planning and record and verify systems. These systems do not appear to be designed with patient dosimetry audit in mind. It is a simple matter to look up the records of individual patients, but these systems are not set up for outputting large-scale patient dose information in the format facilitating audit (such as a spreadsheet). So a solution is needed to avoid manually combing individual patient records one at a time for data, an extremely inefficient proposal.
An initial audit showed that radiographers had been keeping paper records of all CT scan dose information (largely for historical reasons) and so these data were transcribed for analysis. These records did not specify the protocol used but the examination purpose and so there could be issues with naming conventions, with different description actually referring to the same type of scan. Knowing the protocol would allow such cases to be identified and merged for more reliable audit. To help resolve this, a simple in-house software was developed to search the data and extract the protocol information based on keywords in examination information. This allowed protocols to be matched against the manual descriptions and in discussion with radiographers, datasets can be merged where similar protocols are used. The hope in future is that the in-house software will be usable more widely to allow the need for manual transcription and interrogation of the data to be reduced and the data simply extracted.
So as with nuclear medicine CT, we have been able to implement a system for local audit of radiotherapy planning CT (and incidentally demonstrate compliance with NDRLs was being achieved), although with some room for further improvement to automate the process. The challenges were much more practical than analytical in this case, meaning that most of the work involved in setting up local audit is in finding efficient ways to reliably collect data on a large scale and so this may have to be carefully considered when implementing audit elsewhere.
References
1 Ionising Radiation (Medical Exposure) Regulations 2017. Statutory Instruments 2017, No. 1322. London, HMSO.
2 IPEM. Guidance on the Establishment and Use of Diagnostic Reference Levels for Medical X-ray Examinations. Institute of Physics and Engineering in Medicine. Report Number 88, 2004.
3 Hart D, Hillier MC, Shrimpton, PC. Doses to patients from radiographic and fuoroscopic X-ray imaging procedures in the UK – 2010 review. Health Protection Agency. Report Number: HPA-CRCE-034, 2012.
4 Shrimpton PC et al. Doses from computed tomography (CT) examinations in the UK – 2011 review. Public Health England. Report Number: PHE-CRCE-013, 2014.
5 Public Health England, 2016. Diagnostic Radiology: National Diagnostic Reference levels (NDRLs). www.gov.uk/government/publications/diagnostic-radiology-national-diagnos… (published 15 November 2018) (accessed August 2019).
6 Charnock P, Moores BM, Wilde R. Establishing local and regional DRLs by means of electronic radiographical X-ray examination records. Radiat Prot Dosimetry 2013;157:62–72.
7 Charnock P et al. Establishment of a comprehensive set of regional DRLs for CT by means of electronic X-ray examination records. Radiat Prot Dosimetry 2015;163:509–20.
8 Etard C et al. National survey of patient doses from whole-body FDG PET-CT examinations in France in 2011. Radiat Prot Dosimetry 2012;152:334–8.
9 Jallow N et al. Diagnostic reference levels of CT radiation dose in whole-body PET/CT. J Nucl Med 2016;57:238–41.
10 Alkhybari EM et al. Determining and updating PET/CT and SPECT/CT diagnostic reference levels: A systematic review. Radiat Prot Dosimetry 2018;182:532–45.
11 Dennis JL, Gemmell A, Nicol AJ. Optimization of the CT component of SPECT-CT and establishment of local CT diagnostic reference levels for clinical practice. Nucl Med Commun 2018;39:493–9.
12 Connor SO, Mc Ardle O, Mullaney L. Establishment of national diagnostic reference levels for breast cancer CT protocols in radiation therapy. Br J Radiol 2016;89:20160428.
13 Iball GR et al. A national survey of computed tomography doses in hybrid PET-CT and SPECT-CT examinations in the UK. Nucl Med Commun 2017;38(6):459–70.
14 Wood TJ et al. IPEM topical report: the first UK survey of dose indices from radiotherapy treatment planning computed tomography scans for adult patients. Phys Med Biol 2018;63:185008.
15 Gardner M et al. Patient dosimetry audit for establishing local diagnostic reference levels for nuclear medicine CT. Br J Radiol 2017;90:20160850.
16 Bebbington N et al. UK national reference doses for CT scans performed in hybrid imaging studies. J Nucl Med 2016;57(no. supplement 2):594.
Bone and joint infections are an important cause of morbidity and mortality worldwide, particularly in those with inflammatory arthritis. In the US, the prevalence of septic arthritis is 2–10/100,000 but rises to 30–70/100,000 in patients with rheumatoid arthritis. Patients with underlying rheumatological conditions are at an increased risk of infection due to existing abnormal joints (in the case of inflammatory arthritis), as well as immunosuppressive therapies. The British Biologics Register data shows that the risk of patients with rheumatoid arthritis developing septic arthritis doubles in patients treated with biologic therapy compared with non-biologic therapy.1
The classical signs and symptoms of bone and joint infection are joint pain, fever and swelling.
Presentation of bone and joint infections in patients with rheumatological conditions can be variable and more indolent. Correct diagnosis and management can therefore require a higher index of suspicion.
There are two major challenges: overlapping symptoms and an immunosuppressed state.
Overlapping symptoms
A history of pain, swelling and fever as well as classical symptoms of infection could also be due to the patient’s underlying inflammatory arthritis. This can lead to a delay in diagnosis. Even in patients without underlying rheumatological disease, the delay in diagnosis can range from two weeks to nine months.2
A rheumatological flare may affect several joints; however, if only a sterile joint is aspirated for microscopy and culture this could then lead to a missed infected joint elsewhere.
Immunosuppressed state
The other consideration is that both the use of immunosuppressive agents and the underlying inflammatory arthritis can alter the response of the immune system to infection. This makes patients more prone to acquiring an infection; but also once an infection has occurred, can make the symptoms and signs more insidious.
In addition, local administration of corticosteroids, can also be a risk factor for bone and joint infection, particularly when performed in primary care and on patients with rheumatoid arthritis.3 However, the overall incidence of this is low.4
Taking both of these factors into consideration, a thorough history and examination of new joint swelling, joint or back pain and fever, in the case of septic arthritis, and new back pain and fever in the setting of vertebral osteomyelitis (VOM) and a high index of suspicion on the part of the clinician, is required to appropriately investigate and manage the patient.
Obtaining a microbiological diagnosis is the cornerstone of investigation of bone and joint infection.
Microbiological diagnosis
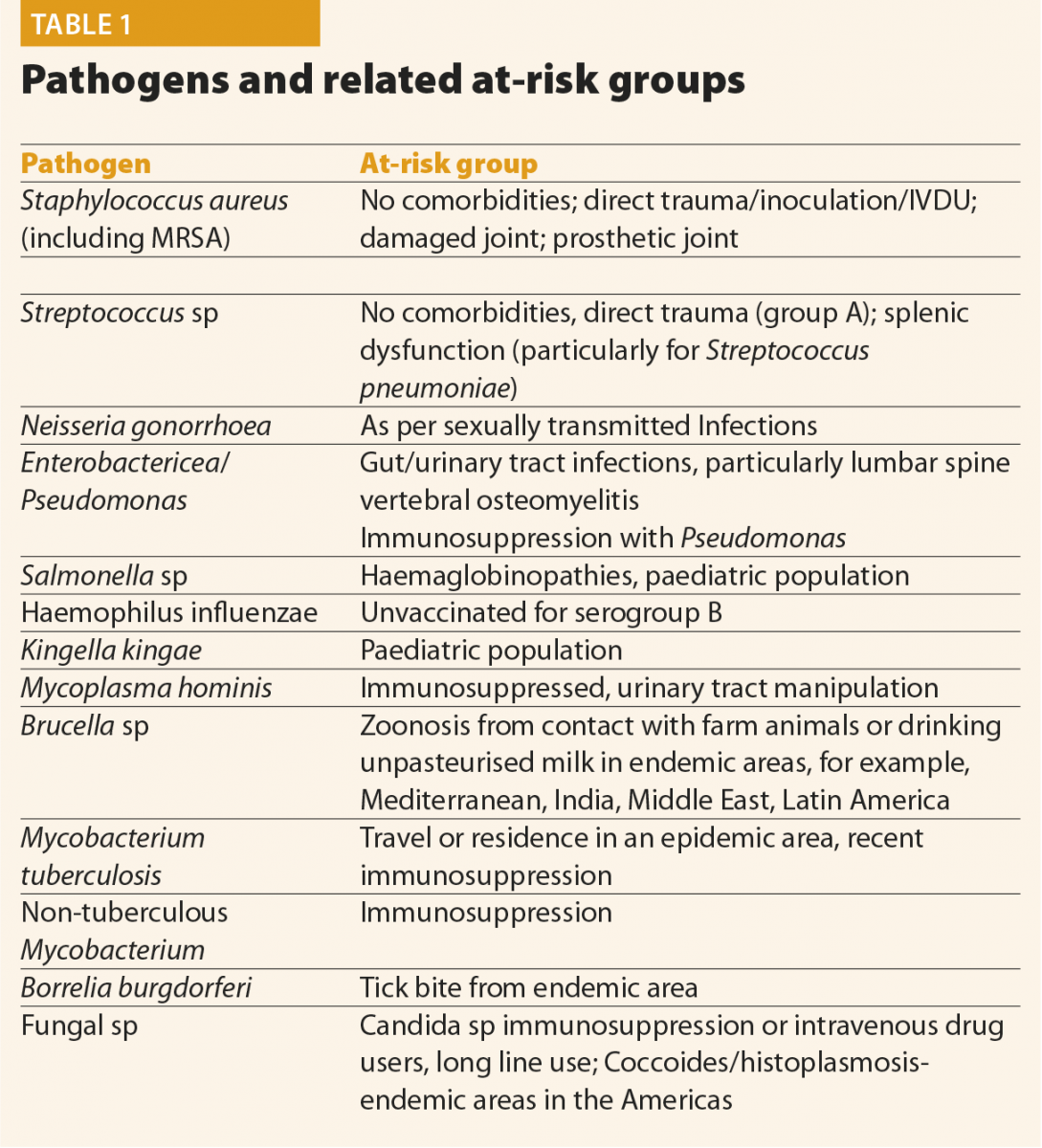
The main pathogens causing septic arthritis and osteomyelitis are bacterial, with Staphylococcus aureus infections in around three quarters of patients being the most prevalent. Pathogens and their risk factors are listed below.
For pyogenic bone and joint infections, appropriate samples to take include fluid aspirate for microscopy, Gram stain and culture, as well as blood cultures. It is important to highlight that Gram stain is positive in only 50% of patients, so for those with a high clinical index of suspicion, antimicrobials should be initiated/continued even with a negative Gram stain result until culture results are available. Intraoperatively, fluid samples in septic arthritis or tissue samples in VOM should also be sent to guide future antimicrobial therapy.
In the setting of culture negative bacterial bone and joint infection, 16sPCR can be used to amplify and identify any bacterial DNA on a sample.5 It is important to understand the limitations of molecular diagnostic tests – for example, a positive sample may still be a contaminant, and there are no commercially available techniques at present to predict sensitivity patterns even if a pathogen is isolated. However, PCR may still be useful if an organism is difficult to culture or if antimicrobials were started before culture was taken.
If tuberculosis is suspected, samples should be sent for Mycobacterial culture, and consideration of PCR for MTB complex (where available). A careful history and Imaging should be performed to elicit any evidence of disseminated tuberculosis. In patients with with suspected Lyme arthritis (that is, risk factors such as a tick bite from an endemic area), investigations should include Lyme serology on serum. Fluid aspirate samples can also be sent for PCR if locally available to aid detection of Borrelia burgdorferi.
Fungal infection is a rare cause of septic arthritis. If fungal infection is suspected, usually in the setting of a highly immunosuppressed patient or intravenous drug user, fluid should be sent for fungal culture. If this culture is negative – further tests could include 18sPCR, panfungal, and fungal markers such as galactamman and beta glucan in blood may be useful. In the setting of appropriate travel history to North and South America, serology for the diamorphic fungi, for example, Histioplasma, Coccoides and Paracoccoides, can also be useful.
Biomarkers
C-reactive protein (CRP) has been the mainstay for detecting and monitoring inflammation and infection for many years. However, there are well described controversies with differentiating between the inflammation of rheumatological conditions and that of infection. There may also be issues with falsely low CRPs in the setting of a patient on DMARDS,6 Cox-2 inhibitors and corticosteroids.
Procalcitonin is a precursor to the hormone calcitonin and rises in response to bacterial infection. Interest in procalcitonin as a specific marker for bacterial infection has increased and there is some evidence to show that it is not affected by corticosteroids.7 A meta-analysis looking at serum procalcitonin in differentiating between septic and inflammatory arthritis showed a specificity of 95% (CI 87–98%) and a sensitivity of 54% (38–62%) compared with serum CRP and a sensitivity of 45% (CI 35–55%) and specificity of 7.9% (CI 2.1–2.5%).8
Unfortunately there is little published data on the effects of DMARDs on other serological biomarkers, for example, IL-1 or PTX-3, and their ability to distinguish between rheumatological inflammation and infection. However, this may be an interesting area for future research, as these markers are not yet commercially available.
Joint fluid biomarkers are also being developed as another method to try to differentiate between inflammation and infection, particularly in the setting of prosthetic joint infection. One systemic review and meta-analysis comparing 13 different tests determined that alpha defensin was the optimal synovial marker and that there may be scope to use others in combination. However the effect of immunosuppressive therapies on this is unknown.9
Radiological investigation
With regards to the radiological diagnosis of VOM, the IDSA10 recommends MRI spine as first-line modality followed by technicium gallium scan CT or PET CT if there are contraindications to MR. MRI has a sensitivity
of 97% and specificity of 93%; however, it is well known that radiological changes can be absent early on in infection and there can be artefact effect in the presence of prosthesis. Gallium scan combined with a bone scan is reported as having a sensitivity of 91% and specificity of 90%.
CT PET is also useful for the diagnosis of osteomyelitis and detecting metastatic infection; however, it would be difficult to differentiate between increased uptake due to inflammation, malignancy or infection. Nevertheless, there is evidence to show that CT PET is more sensitive and specific in the setting of chronic vertebral osteomyelitis than labelled WCC. CT PET has also been evaluated and found to be useful in serial scans in both the post-surgical setting and in the general follow up of patients, whereas with MRI, it is known that radiological change can lag behind clinical cure.
Unfortunately data evaluating this modality in the setting of the rheumatological patient are lacking, and the obvious concern would that be the areas of increased uptake could be due to either the underlying inflammatory disease or infection.
Septic arthritis of a native joint can be life-threatening and cause permanent joint damage if left untreated. In most cases, surgical washout is required, as well as appropriate antimicrobials, which might need to be initiated before a joint aspirate is taken if there is going to be a significant delay. Empirical antimicrobials are based on local sensitivities, patient risk factors (for example, allergy) and pharmacokinetics and dynamics (such as bone penetration and patient’s renal function). In general terms, empirical coverage is aimed at Staphylococcus aureus and Streptococcus species as these are the most common causes of infection. After appropriate source control the duration of antimicrobials of a native joint is usually two to four weeks.
If clinical suspicion of infection remains high, but initial bacterial culture is negative, cases should be discussed with an infection specialist to guide ongoing testing. This is particularly important for those with risk factors for more unusual pathogens, as listed in Table 1.
For pyogenic vertebral osteomyelitis, if the patient is not septic and there is no neurological compromise (either clinically or radiologically), the initiation of antimicrobials can be deferred until appropriate cultures are taken and a microbiological diagnosis is confirmed.10 Cultures to be taken include blood cultures and, where amenable, a biopsy. There are no clinical studies regarding duration of antimicrobials in patients with rheumatoid arthritis but a recent study has shown non-inferiority of six weeks’ duration of antimicrobials compared with 12 weeks in pyogenic infection.11
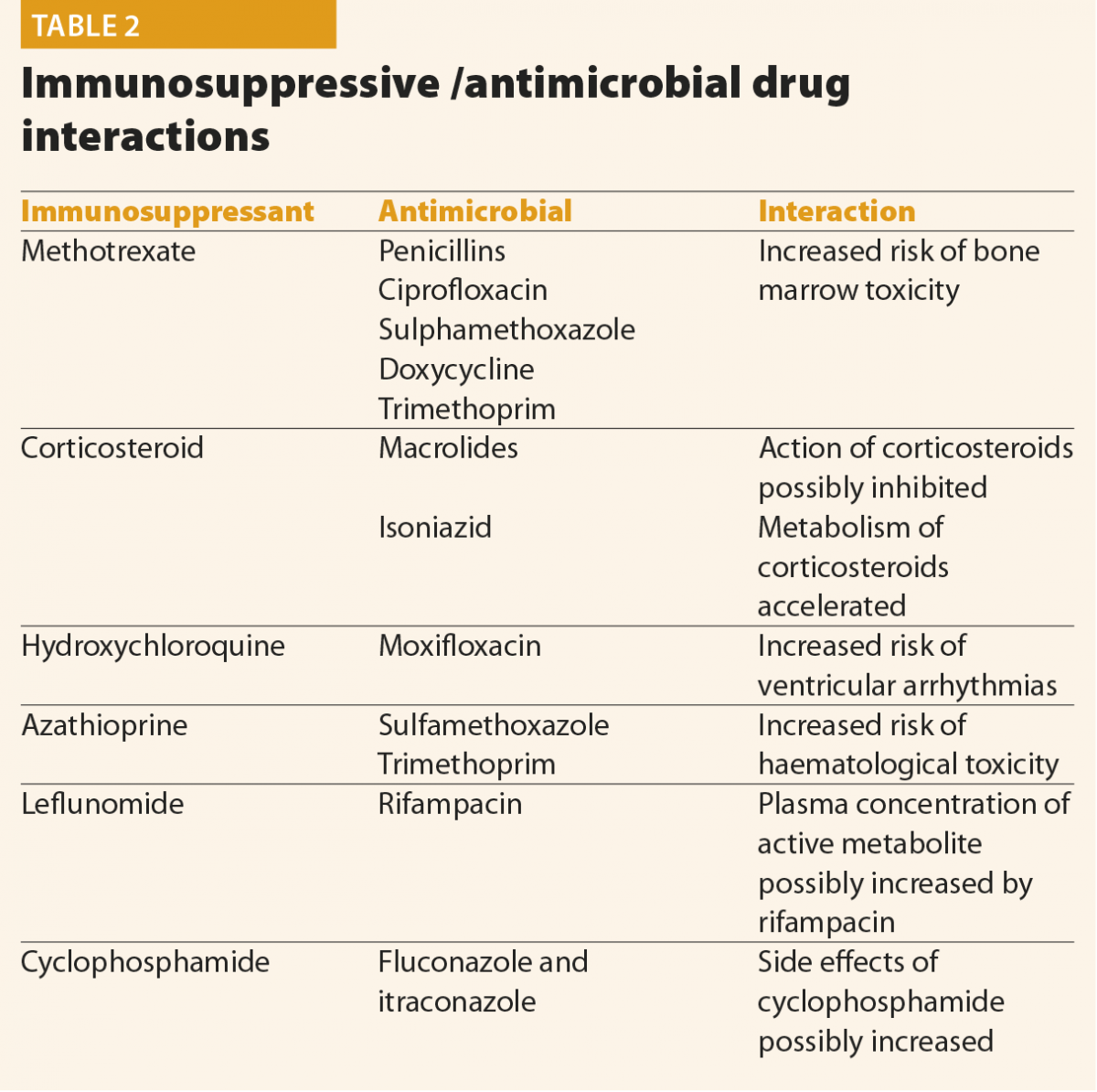
The type of antimicrobial depends on the pathogen and sensitivities. However, in patients with inflammatory arthritis who may be on a number of medications, a careful assessment of drug interactions and their risk versus benefit should be assessed. Common interactions are listed in Table 2. This is not an exhaustive list and a risk–benefit analysis needs to be made with a rheumatologist, infection specialist and the patient as to which chosen antimicrobial regimen is the most safe and most potent. Other considerations specific to the rheumatology patient in the face of an invasive infection is whether immunosuppressive medications should be reduced or stopped and, more importantly, when these should be restarted. Interestingly a recent meta-analysis in the paediatric, non-rheumatological population has shown favourable outcomes in corticosteroid use in septic arthritis, although it should be highlighted this might not be generalisable to the adult rheumatological population.12
The assessment, investigation and management of a patient with bone and joint infection and inflammatory arthritis is complex and requires a multidisciplinary approach. This should include rheumatology, infectious diseases, radiology and orthopaedics with expertise and special interest in managing the challenges with which this cohort of patients present.
More research is required into specific biomarkers and radiological modalities for this group of patients in order to deliver high quality and evidence-based care. It is likely that the prevalence of infection in rheumatological patients with complex infection will continue to rise with increasing use of immunosuppressives.
References
1 Galloway JB et al. Risk of septic arthritis in patients with rheumatoid arthritis and the effect of anti-TNF therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2011;70(10):1810–14.
2 Beronius M et al. Vertebral osteomyelitis in Göteborg, Sweden: a retrospective study of patients during 1990–95. Scand J Infect Dis 2001;33:527–32.
3 Xu C et al. Risk factors and clinical characteristics of deep knee infection in patients with intra-articular injections: A matched retrospective cohort analysis. Semin Arthritis Rheum 2018;47:911–16.
4 Wang Q, Jiang X, Tian W. Does previous intra-articular steroid injection increase the risk of joint infection following total hip arthroplasty or total knee arthroplasty? A meta-analysis. Med Sci Monit 2014;20:1878–83.
5 Woo PC et al. Then and now: use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin Microbiol Infect 2008;14:908–34.
6 Mysler E et al. Influence of corticosteroids on C-reactive protein in patients with rheumatoid arthritis. Arthritis Res Ther 2004;6(Suppl 3)57:1–41.
7 Perren A et al. Influence of steroids on procalcitonin and C-reactive protein in patients with COPD and community-acquired pneumonia. Infection 2008;36(2):163–6.
8 Zhao J et al. Serum procalcitonin levels as a diagnostic marker for septic arthritis: A meta-analysis. Am J Emerg Med 2017;35(8):1166–71.
9 Lee YS et al. Synovial fluid biomarkers for the diagnosis of periprosthetic joint infection: A systematic review and meta-analysis. J Bone Joint Surg Am 2017:99:2077–84.
10 Berbari E et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 2015;61(6):e26–e46.
11 Bernard L et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 2015;385(9971):875–82.
12 Farrow L. A systematic review and meta-analysis regarding the use of corticosteroids in septic arthritis. BMC Musculoskelet Disord 2015;16(241):1–8.
Infection following prosthetic joint infection (PJI) is a recognised surgical complication. However, it has serious consequences requiring both surgical and antimicrobial management. Patients with rheumatoid arthritis (RA) are both more likely to have joint replacement due to abnormal anatomy and be immunosuppressed due to the underlying disease and immunomodulatory therapies. These factors make them more at risk as a population for post- operative infection.
Due to progressive joint destruction, patients with RA commonly have to undergo joint replacement. It is estimated that 24% of patients with RA will eventually require their first major joint replacement at 16–20 years after diagnosis.1 Of those who have a total hip or knee replacement 5–7% have RA compared to 1% in the general population.2 There is an increased risk of PJI due to the underlying RA which can be 2.5-times that of a person with osteoarthritis.2
In the general population, infection in PJI can occur from direct seeding peri- or post-operatively or haematogenous spread or recurrence of existing infection from previous prosthesis. The prosthetic material allows the bacteria to form a protective glycocalyx layer or biofilm. This means the bacterial colonies deep within this are protected from host defences and antimicrobials. There may also be poor wound healing and poor host defences due to the underlying inflammatory arthritis in patients with RA. This, coupled with immunomodulatory therapy, makes a favourable environment for infection.
According to the International consensus meeting in 2013 on peri-prosthetic joint infections, the diagnostic criteria for PJI is as follows.3
Major
Minor
A patient is required to have one major or three minors to be diagnosed. However, in a patient with RA, there are limitations to these criteria.
Initial investigation of prosthetic joint infection in patients with RA is similar to other patients. A careful history and examination should be taken to ellicit:
A) Local features of infection joint swelling, pain, wound breakdown and sinus tract
B) Past medical and surgical history, including previous revisions, microbiology and previous antimicrobial treatment, previous limb cellulitis and other prosthetic material
C) Medication and drug allergy.
There should also be clear documentation on the use of immunosuppressants, acute prescriptions of corticosteroids and infusion therapy that may not be listed on patients’ regular medications as this can affect the immune system up to 12 months after administration.
Full blood count, urea and electrolytes, CRP, ESR and liver function tests should also be part of the standard work up. However, in patients who have RA it might be difficult to differentiate between raised inflammatory markers due to infection or underlying inflammatory arthritis flare. CRP may also be falsely low due to use of immunomodulatory therapies. White blood cells can be elevated due to corticosteroid use.
Other biomarkers specific for bacterial infection, for example, procalcitonin, interleukin-6 (IL-6) and tumour necrosis factor, are in development. A prospective study looking at the sensitivity and specificity of these in prosthetic joint infection identified a combination of IL-6 and CRP as the most useful and that procalcitonin was the most specific.4
There has also been increasing interest in using joint fluid biomarkers to aid in the diagnosis of PJI (for example, CRP, adenosine deaminase (ADA), alpha-2-macroglobulin) which show some promise increasing the positive predictive value in diagnosing PJI vs aseptic loosening.5 Leucoesterase dip stix are easy to use and commercially available but again could also be positive due to underlying RA rather than infection. However none of these biomarkers have been evaluated specifically in patients with inflammatory arthritis with PJI or in patients on immunomodulatory agents. This remains an area for potential research.
With regards to radiology, plain X-rays may be useful for operative planning and for demonstration of loosening of the prosthesis but have a low specificity and sensitivity in differentiating septic and aseptic osteolysis. Plain CT may be used in determining periosteal reaction or soft tissue accumulation. MRI is the most sensitive modality for detection of pus but is limited by its cost and the time the scan takes. Both MRI and CT can be limited by metal artefacts in patients with prosthetic joints. Nuclear medicine scans and FDG PET are useful tools for detection of infection. However, in a patient with RA, there may be difficulties determining the difference between inflammatory arthritis and an infected joint.
The most common pathogens in PJI are Staphylococcus aureus and Coagulase-negative Staphylococcus. However, other bacteria (for example, Streptococcus, Enterococci, Diptheroids, Gram negatives and Anaerobes) can also frequently cause infection. Moreover, PJI infections are often polymicrobial, which can make antimicrobial therapy challenging. The incidence of polymicrobial infection in RA in one cohort was 15%.6 Pathogens causing infection are broadly similar in patients with and without RA – perhaps with a higher incidence of Staphylococcus aureus than the general population.6,7
Obtaining a microbiological diagnosis is key to securing optimal treatment. It is recommended that a deep joint aspirate is collected aseptically – ideally in theatre by a specialist to prevent contamination and further infection of the joint. It should be Gram stained and cultured. If the patient is febrile blood cultures should be taken. Ideally cultures should be collected before commencing systemic antibiotics to increase culture sensitivity.
If atypical organisms are suspected, for example where a history suggests tuberculosis, the microbiology lab must be alerted so that appropriate culture methods can be used and laboratory safety precautions taken.
Mycobacterial infection should be investigated and managed by an experienced physician, looking for a history of exposure or previous infection and examination and imaging for disseminated infection. Fluid should be sent for mycobacterial culture and Mycobacterial polymerase chain reaction (PCR).
If the patient is felt to be at risk of fungal infection, fluid and blood cultures should be sent with fluid being cultured on fungal selective agar. Blood markers such as beta glucan and Aspergillus antigen, if available locally, should be sent. These cases should be discussed with local Infection specialists to ensure correct specimens are sent.
In the setting of culture-negative PJI, that is, one where no pathogen has been isolated, the patient should be evaluated for the likelihood for a less typical pathogen. There should also be discussion with an infection specialist about empirical antimicrobial therapy, which is usually directed at Staphylococcus species. Tissue samples can also be sent for 16S and 18S PCR analysis to improve sensitivity of detection of bacteria and fungi respectively. This may be useful if the sample was taken on antimicrobials. The limitations with PCR can be availability, possible contamination (and therefore detection of organism that is not the infecting pathogen) and the lack of antibiogram data.
Histological analysis can be used for the diagnosis of PJI. A systemic review of intra-operative frozen sections demonstrated it was a very good predictor of culture positive joint infection and moderate at predicting culture negative cases based on the presence or not of acute inflammation, that is, >5 PMN in at least five separate high powered fields.8 Stains can also be performed for fungi or mycobacterial infection. However there was no subgroup analysis of patients with inflammatory arthritis and whether this affects histological diagnosis. The presence of acute inflammation may not be present in less virulent organisms, for example, Cutibacterium acnes.
The management of PJI in general comprises both medical and surgical options with a multidisciplinary team including orthopaedic and plastic surgeons, infection specialists and rheumatologists. Conventional surgical options for the infected joint replacement are retention and debridement, one or two stage revision procedures, resection of arthrodesis or, as a last resort, amputation.
During surgery, multiple (five to six) deep samples should be taken for Gram stain and culture, each with fresh sterile instruments and without passing through a sinus in order to prevent contamination.
Sonicade and bead milling are newer intraoperative techniques to increase culture sensitivity.
Sonicade involves placing bone tissue in sterile water then passing ultrasound waves through this media. It has been shown to increase the sensitivity of culture and also PCR detection.9
Bead mill processing involves grinding of bone fragments and then processing in blood culture and agar which appear to decrease turnaround times.
There have been several studies looking at the outcomes in surgical management in patients with RA.6,7 One retrospective study looking at 246 episodes of PIJ in patients with RA showed that as per the general population, a two-stage procedure was associated with the best outcome and highlighted that not using antimicrobial impregnated cement was associated with risk of re-infection.7 For all the reasons mentioned, the risk of re-infection of the prosthetic joint is unsurprisingly higher in patients with RA-up to 25% compared with 5% in the general population in both of these studies even with a two-stage procedure. Both studies showed contrasting results with regards to immunosuppressive therapies and the influence on outcomes, leaving this an area for future research. Ultimately any non-randomised control study looking at surgical outcomes in this field is limited by the fact that patients undergoing a two-stage procedure are more likely to have favourable joint anatomy and have fewer comorbidities. This reflects the fact that the final operative management rests with the surgical team.
With regards to medical or antimicrobial therapy of PJI infections this is highly individualised by the micro-organisms and their susceptibilities. Every patient with PJI should be reviewed by an infection specialist. The IDSA10 recommends two to six weeks of pathogen specific therapy in combination with oral rifampicin in susceptible isolates with follow-on oral therapy for three months in total hip replacement and six months in total knee replacement. It also states that IV therapy should go on for four to six weeks if rifampicin cannot be used, but evidence is lacking and this is not our local practice. Lifelong suppressive antimicrobials may be considered if removal or further revisions cannot be performed. With regards to a patient with RA and PJI, the specific medical management might also be affected by drug interactions with immunosuppressive therapy and decisions must be made within a skilled specialist multidisciplinary team.
Other important medical management considerations in PJI infection in RA is the discontinuation of immunosuppressive therapy in elective patients admitted for revision arthroplasty. This should be performed in conjunction with a rheumatologist, especially when considering stopping long-term corticosteroids due to the risk of developing acute adrenal insufficiency. The international consensus meeting on peri-prosthetic joint infections defined timings based on half-lives for stopping immunomodulatory therapy for elective joint replacement. This remains an area for research in patients with PJI. The decision to stop, reduce or continue with the current immunosuppression will require an individualised risk vs benefit analysis.
As with diagnosis, follow up and response to medical and surgical of PJI in RA can be challenging. The joint might be inflamed and biomarkers may be persistently raised due to the patients underlying inflammatory condition rather than persistent infection.
PJI infection in RA remains more common than in the general population and is challenging to diagnose and manage. Treatment should be individualised under a multidisciplinary team in a specialised centre. Areas of future research to provide a better evidence base include the effect of RA and immunomodulatory therapies on the newer specific bacterial biomarkers and the effect of immunosuppressive therapies on recovery from PJI infection.
References
1 Kapetnovic MC et al. Orthopaedic surgery in patients with rheumatoid arthritis over 20 years: prevalence and predictive factors of large joint replacement. Ann Rheumatol Dis 2008:67;1412–16.
2 Berbari EF et al. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis 1998;27:1247–54.
3 Parvizi J at al. Definition of periprosthetic joint infection. J Arthoplasty 2014;29(7):1331.
4 Bottner F et al. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg 2007;89:94–9.
5 Sousa R et al. Improving the accuracy of synovial fluid analysis in the diagnosis of prosthetic joint infection with simple and inexpensive biomarkers: C-reactive protein and adenosine deaminase. Bone Joint J 2017;77:351–77.
6 Berbari EF et al. Outcome of prosthetic joint infection in patients with rheumatoid arthritis: the impact of medical and surgical therapy in 200 episodes. Clin Infect Dis 2006;42:216–23.
7 Hsieh PH, Huang KC, Shih HN. Prosthetic joint infection in patients with rheumatoid arthritis: an outcome analysis compared with controls. PLoS One 2013;8:e71666.
8 Tsaras G et al. Utility of intraoperative frozen section histopathology in the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am 2012;94:1700–11.
9 Trampez A et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med 2007;357:654–63.
10 Osmon DR et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013;56:1–10.
Globalisation affects all areas of life, including healthcare. For globalisation to have a positive influence, it is necessary to have global standards and units to which all measurements are traceable. We are familiar with global standards for time, temperature, mass and length and these standards, coupled with Système Internationale (SI) units, ensure that these parameters can be measured anywhere in the world with a small measurement uncertainty, enabling the safe transfer of data between global stakeholders.
The same principle should apply to measurements made, and results used, in healthcare.
Laboratory medicine is an essential clinical specialty providing users with pivotal information for the prevention, diagnosis, treatment and management of health and disease. Laboratory medicine has several specialisms including clinical chemistry, genetics, haematology, immunology, microbiology and transplantation. Laboratory medicine results provide information that impacts a high percentage of clinical decisions in healthcare. This central role means that laboratory medicine specialists have a professional responsibility to provide a high-quality service that is optimised to the needs of the patient.1
A high percentage of laboratory medicine results are obtained using commercially produced in vitro diagnostic (IVD) test systems. The global market for these IVD products was estimated at $61 billion in 2016, with a growth rate of 4.6% per annum.2 Many companies are involved in producing IVD test systems. Unfortunately, it is common for the results obtained from different IVD test systems for the same analyte to be unacceptably high. Therefore, one increasingly important quality objective is to ensure that patient test results are traceable (equivalent) between different methods, laboratories and healthcare systems over time.3
Equivalence of test results is being achieved through the process of harmonisation in laboratory medicine. The aim of harmonisation is to provide accurate, actionable and transferable patient results, which can facilitate improved clinical outcomes and patient safety. Harmonisation in laboratory medicine has a wide scope. It can be applied across the total testing process of laboratory medicine, including requests, samples, measurements and reports.4 The many dimensions of harmonisation require active involvement at local, national and international levels.5
There are several reasons why efforts should be made to reduce between-method variability in laboratory medicine.4 These include:
Patients and the public naturally assume that all methods for measurement of a single analyte will give the same result on a patient sample. For some simple analytes, such as plasma glucose, the results will be very similar. However, for more complex analytes, the results might vary considerably. There are many potential reasons for these differences, and these can be summarised as follows:
Metrology is the science of measurement. The application of metrology provides the key to reducing measurement variability by facilitating the adoption of international reference systems to enable alignment from different methods. The increasing application of metrological traceability in laboratory medicine (TLM) to underpin those reference systems is reducing the variables that are responsible for methods giving different results.6 TLM is an important area of laboratory science that is often poorly understood. Achieving TLM is a global multi-stakeholder, cooperative activity involving metrologists; international standards organisations; scientific and clinical experts from international professional bodies; healthcare regulators; and the IVD industry.7
Metrological traceability is defined as the property of a measurement result, which can be related to a reference standard through a documented unbroken chain of calibrations.
The principles of a reference measurement system for establishing metrological traceability are described in the relevant International Standards Organisation (ISO) document.8 The components of a reference measurement system comprise reference materials (calibrators) and reference measurement procedures (methods), both of which exist at different hierarchical levels.
The inter-relationship between the components of a reference measurement system may be described in the metrological traceability chain.8
Figure 1 depicts this traceability chain with higher order reference materials and measurement procedures at the top and lower order towards the bottom. At the top of the chain is the definition of the measurand, the substance intended to be measured, expressed in SI units. The hierarchy of reference materials and measurement procedures is depicted by the rising metrological traceability arrow. Descent through the traceability chain is accompanied by increasing measurement uncertainty as depicted by the downward arrow. Figure 1 also depicts the contributions to TLM of the metrology institute or reference laboratory; the IVD method manufacturer; and the routine service laboratory.
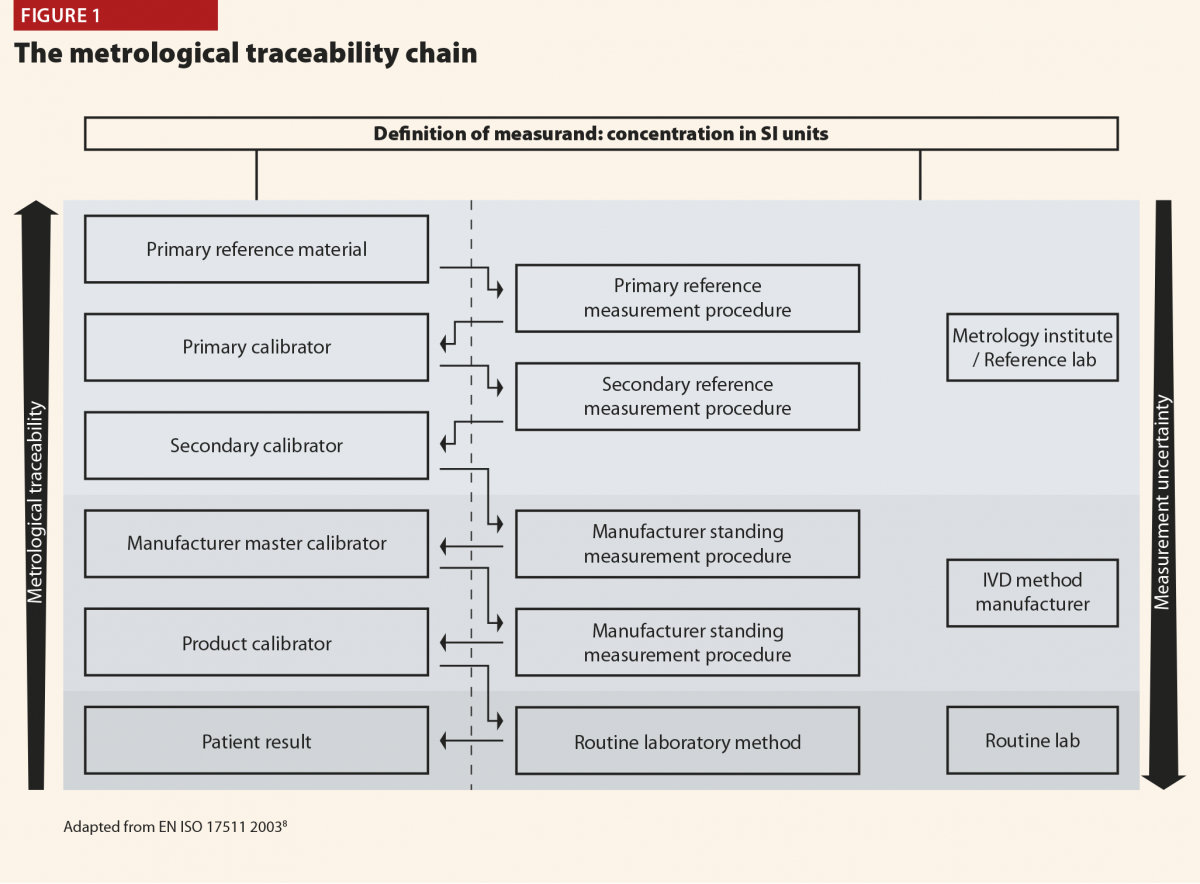
The relevance of TLM may be explained by considering the measurement of plasma glucose, one of the most common biomarker tests. There are many IVD methods for plasma glucose, but they all comply with the traceability chain so that the result obtained in the routine laboratory can be traced back to a primary reference material of pure glucose. As a result, there is excellent agreement between methods for plasma glucose, and results from elsewhere can be interpreted with confidence.
For structurally simple molecules, like many of those measured routinely in clinical chemistry, pure substance is available, and it is possible to have a complete unbroken traceability chain. Even for some protein molecules it is possible to achieve full metrological traceability by using a unique, signature peptide as the primary reference material. Consequently, there are a growing number of important biomarkers where method standardisation has been achieved in a way comparable to plasma glucose.
Regrettably, most of the biomarkers measured in laboratory medicine are not structurally simple molecules. For example, complex proteins, nucleic acids, viruses and bacteria are clinically important biomarkers that may not be available as pure substance and reference methods of measurement are unlikely to be available. In these circumstances full metrological traceability is not possible. The adoption of international conventional calibrators, with values assigned by experts, represents traceability part of the way up the chain and the use of such calibrators provides a reference against which methods can be harmonised to reduce between method variability.
The JCTLM was established in 2002 through a declaration between the International Bureau of Weights and Measures (BIPM), the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) and the International Laboratory Accreditation Cooperation (ILAC) in response to the implementation of the European Community Directive 98.79/EC on in vitro medical devices. JCTLM currently has >50 members from 20 countries and its membership is growing rapidly.
The aim of the JCTLM is to support world-wide comparability, reliability and equivalence of measurement results in laboratory medicine, for improving health care and facilitating national and international trade in IVD devices, by:
The main output of the JCTLM is the global database of higher order reference materials; reference measurement methods/procedures; and reference measurement services.9 This database is freely available to any user. In July 2019, the JCTLM database contained entries for some 300 reference materials and almost 200 reference methods. The supporting output of the JCTLM is a website that contains freely available educational support materials, including webinars, publications and presentations from meetings.10
As the content of the JCTLM database demonstrates, significant progress has been made in the application of TLM to produce reference materials and measurement procedures. However, more than 1000 analytes are routinely measured across laboratory medicine, and there are significant challenges in implementing global TLM for many of these analytes. These challenges include:
These challenges are being addressed but progress is largely dependent on voluntary effort and so it is too slow to meet the needs of patients. A ‘call to arms’ has been issued to establish a global forum to manage TLM.11
While much of the effort involved in achieving TLM is a global endeavour, there are steps that can be taken by hospital and laboratory managers to protect patients and to educate the laboratory users for whom they are responsible. These steps include:
References
1 Beastall GH. Adding value to laboratory medicine: a professional responsibility. Clin Chem Lab Med 2013;51:221–8.
2 IVD market overview. Allied Market Research 2018. www.alliedmarketresearch.com/ivd-in-vitro-diagnostics-market (accessed August 2019).
3 Greenberg N. Update on current concepts and meanings in laboratory medicine – standardization, traceability and harmonization. Clin Chim Acta 2014;432:49–54.
4 Plebani M. Harmonization in laboratory medicine: the complete picture. Clin Chem Lab Med 2013;51:741–51.
5 Plebani M. Harmonization in laboratory medicine: requests, samples, measurement and reports. Crit Rev Clin Lab Sci 2016; 53:184–96.
6 White GH. Metrological traceability in clinical biochemistry. Ann Clin Biochem 2011; 48: 393-409
7 Beastall GH, Brouwer N, Quiroga S, Myers GL. Traceability in laboratory medicine: a global driver for accurate results for patient care. Clin Chem Lab Med 2017;55:1100–08.
8 ISO 17511: 2003 In vitro diagnostic medical devices – measurement of quantities in biological samples – metrological traceability of values assigned to calibrators and control materials ISO, Geneva, Switzerland; 2003.
9 JCTLM. JCTLM database of reference materials and measurement procedures. www.bipm.org/jctlm/ (accessed August 2019).
10 JCTLM. Working Group on traceability, education and promotion.www.bipm.org/cc/JCTLM/Allowed/2015-11-30/3_G-Beastall_JCTLM-WG-TEP_2015.pdf (accessed August 2019).
11 Cobbaert C, Smit N, Gillery P. Metrological traceability and harmonization of medical tests: a quantum leap forward is needed to keep pace with globalization and stringent IVD-regulations in the 21st century. Clin Chem Lab Med 2018;56:1598–602.
Parkinson’s disease (PD) is a progressive mobility disorder caused by destruction of dopaminergic neurones within the central nervous system.
A considerable body of evidence is available that demonstrate patients with this disease are at high risk of complications1,2 such as falls or collapses,3 septic infections,4 and dysphagia leading to aspiration,5 as well as high risks of deterioration of mobility and functionality requiring secondary care input.
Timely availability and administration of anti-Parkinson’s medications (APMs) has been highlighted by both Parkinson’s UK6 and the National Patient Safety Association (NPSA)7 as a key implementation in preventing further morbidity in this patient group in secondary care.
EDs are acknowledged to be high risk areas for medication error8–10 with dose omissions of critical medications (including APMs) being highly prevalent.11 Furthermore, considering the current stress on National Health Service (NHS) EDs, patients experience considerable waits both to be seen and a for transfer to base ward level if required. The Royal College of Emergency Medicine, in a statement in 2014, explained that around 500 deaths within EDs could be attributable to overcrowding.
Higher-risk medical patients, including patients with PD, have previously been found to experience longer waiting times in the ED and longer total inpatient stays than other patient populations.12
The clinical presence of pharmacists within inpatient areas in improving quality and safety is now well established.13 Pharmacists are identified as key healthcare professionals in improving medicines optimisation within secondary care. Lord Carter14 was also keen to identify the importance for new and innovative practices required in EDs, to support patient safety and aid with flow.
A preliminary service evaluation15 of a pilot pharmacy service in the ED established the important potential for clinical pharmacy services to prevent dose omissions and improve safety in patients with PD within a large teaching hospital ED. A total of 52 ‘serious’ interventions were recorded over the service period, specifically in relation to APMs (8.8% of the total interventions recorded). Further literature16 explored the potential benefits of clinical pharmacy services in the ED, finding them equally effective at improving safety and quality, as well as reducing ED clinician workload.
This follow-on hypothesis generating study will identify the prevalence of APM dose omission within a large teaching hospital ED, and determine the effect of pharmacist intervention in the care of these patients within the emergency department prior to admission to the ward. We will further explore the role of a pharmacist clinical intervention in these patients, and aim to assess any impact on their care, particularly in the case of missed doses.
A retrospective audit was designed, in order to capture all patients with PD presenting to Nottingham University Hospitals NHS Trust Emergency Department between 1 June 2016 and 31 July 2016.
All patients were included who had a formal diagnosis of PD at the time of presentation to the ED and took at least one medication directly used to treat PD. Patients who were discharged directly from the ED (that is, did not require an inpatient stay) were excluded.
Patients with PD were determined by free text search through electronic records of all presentations between these dates. Key word searches for any presence of ‘Parkinsons’, ‘Parkinson’s’, ‘Parkinsonsism’ ,‘Sinemet’, ‘Madopar’, ‘Co-Beneldopa’, ‘Cobeneldopa’, ‘Co Beneldopa’, ‘Co-Careldopa’, ‘Cocareldopa’, Co Careldopa’, ‘Rotigotine’, ‘Stalevo’, ‘Amantadine’, ‘Rasagaline’ and ‘Selegeline’ were included for further analysis.
Cases identified were then reviewed by a research pharmacist and/or research ED clinician for inclusion under the aforementioned criteria.
For all included cases, the total doses due within ED and the first 24 hours of inpatient admission were collected. This was determined against expert medicines reconciliation by inpatient pharmacists (which was regarded as gold standard). All ED documentation (including drug charts, temporary charts and electronic notes) were reviewed for documentation of medications being administered, delayed or omitted. A lack of documentation was regarded as an omission. Further data around deterioration of symptoms after admission to ED, whether APMs were prescribed in ED, and errors in prescribing APMs were also collected.
Intervention by a pharmacist within the ED, defined by review and confirmation of primary drug history, supply (or prompted administration) of medication and ward handover was also collected for all patients. A single pharmacist was available during the service period, working 07:30-15:30 during weekdays.
Statistical analysis was conducted using Microsoft Excel 2010.
A total of 177 patient records were returned from the key word search, 62 patients were excluded because they were either discharged directly from the ED or did not wait for treatment. A further 23 patients were excluded as records indicated they did not suffer from PD. Lastly, three patients were excluded because complete records were unavailable for analysis; this left a total of 89 patients who met the inclusion criteria and for whom records were available for analysis.
Baseline characteristics (age and gender) were approximately similar in the analysis groups (Figure 1). A total of 84% of patients had at least three documented co-morbidities, with 50% having greater than five (Figure 2a and 2b).
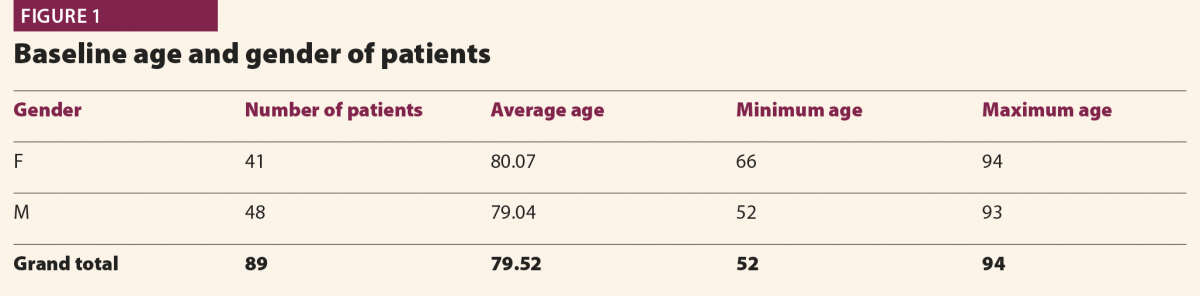
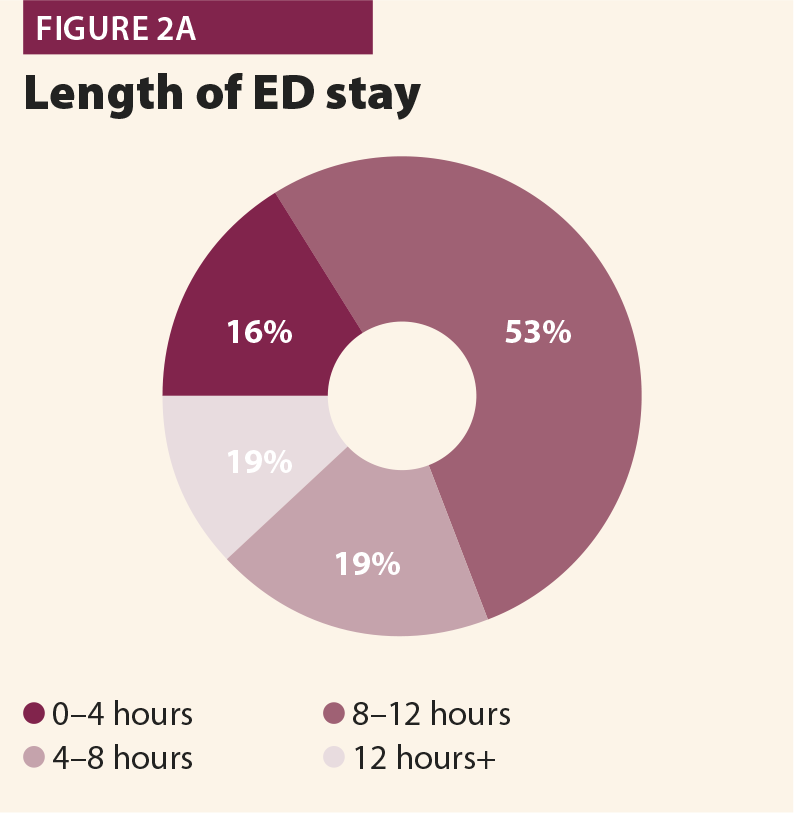
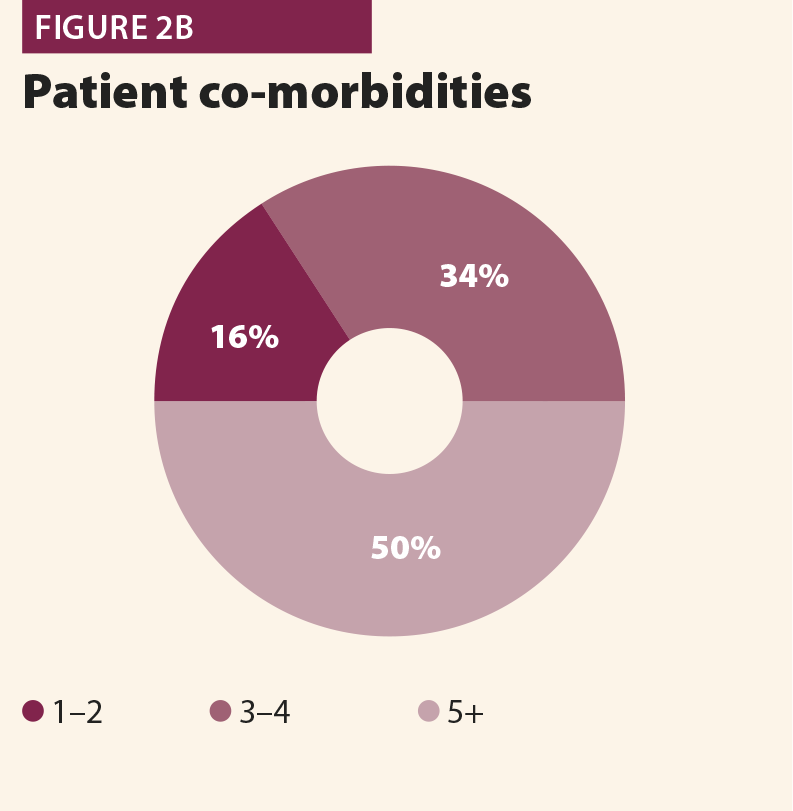
A total of 86.5% of patients were correctly identified within medical clerking in the ED as having PD, and taking oral therapy for the condition; 77.5% were actually prescribed these APMs within the department (Figure 3).
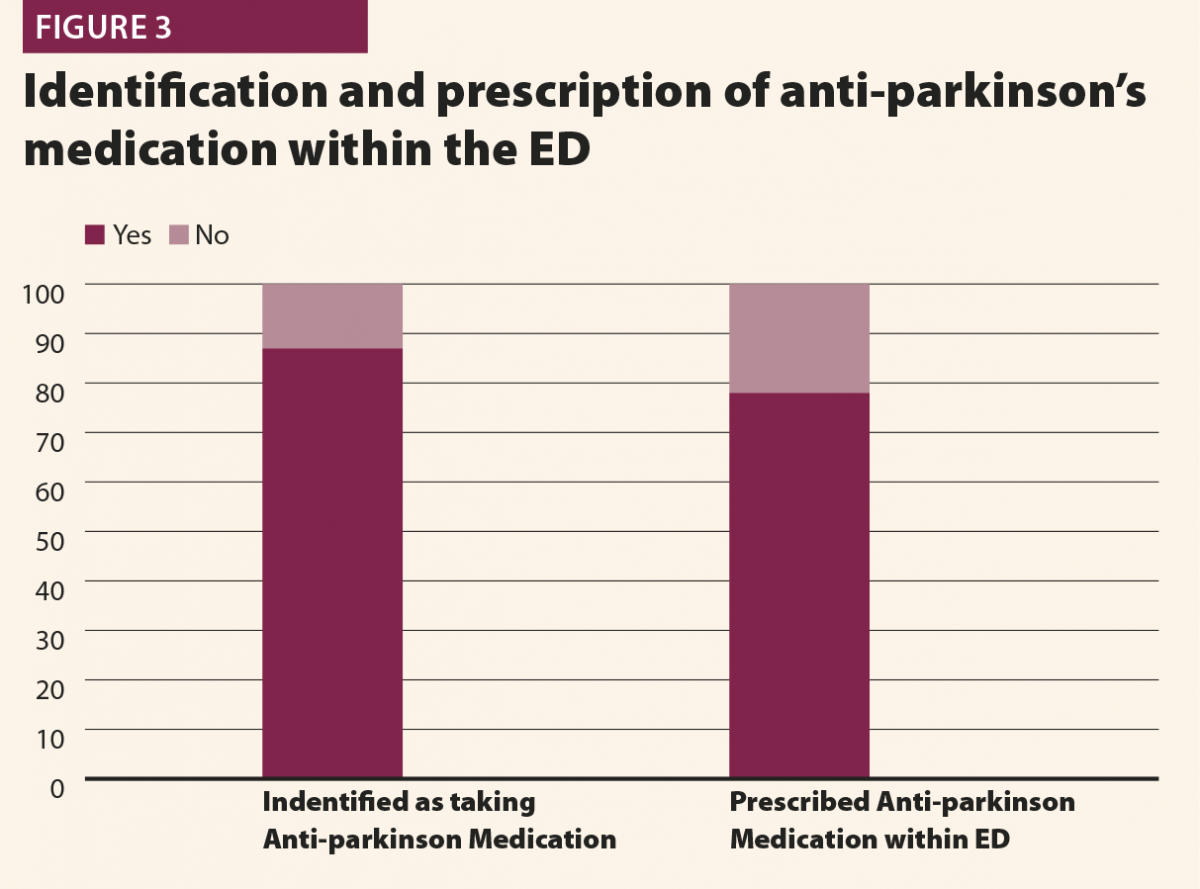
Pharmacist intervention as outlined in the Methods section occurred in 19 of the 89 cases (21.3%). The mean numbers of doses required per patient were similar in both groups.
Dose omissions and delays within the ED were considerably higher in patients who did not have an early pharmacist intervention (Figure 4). This effect was also extended to dose omission and delays on base ward level within the first 24 hours (Figures 5 and 6). The ED pharmacist intervention demonstrated a statistically significant reduction in dose omissions and delays, both while patients were waiting within ED, and also at base ward level.
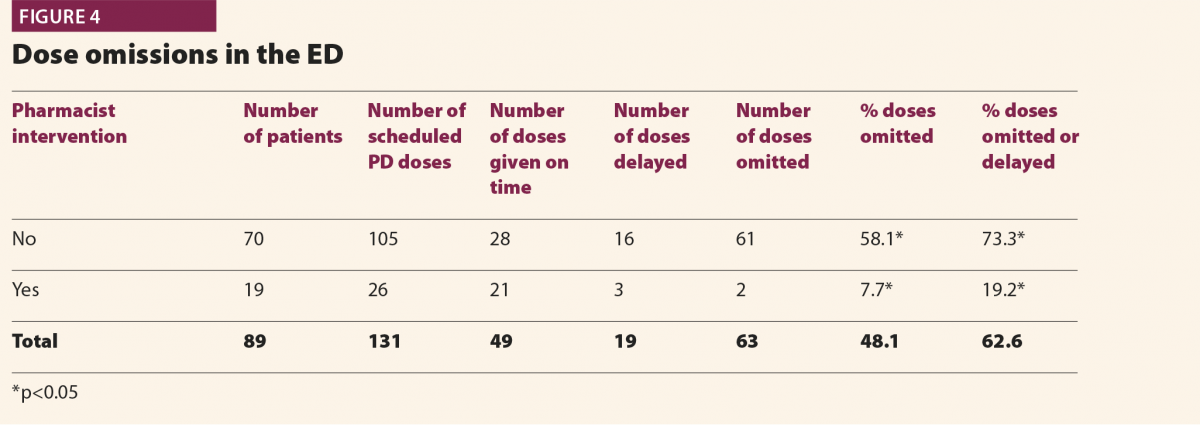
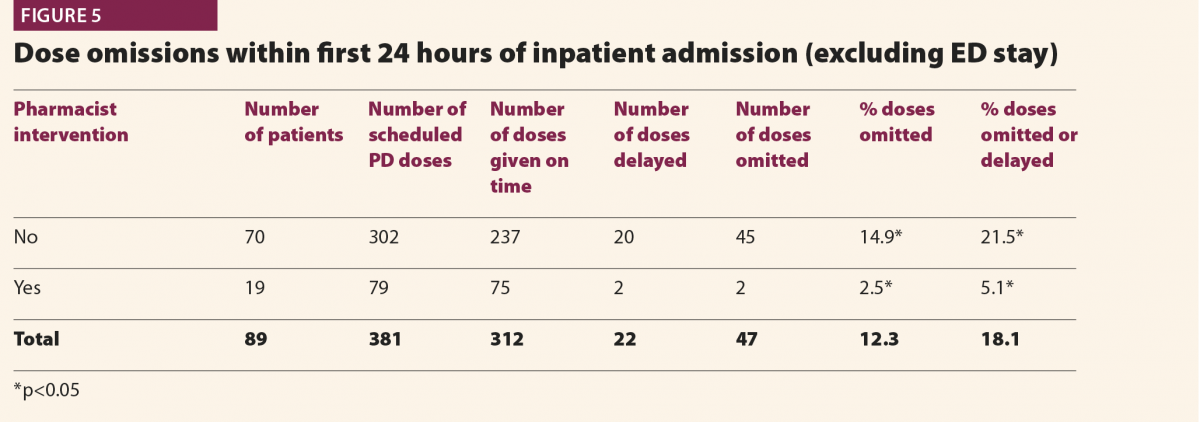
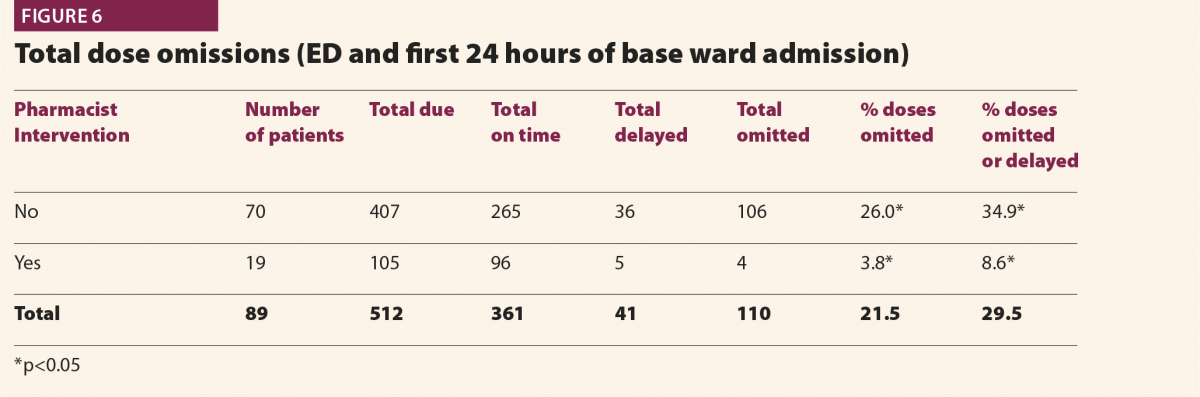
Further analysis around prescribing of APMs within ED, demonstrated an impact on the number of dose omissions at base ward level within the first 24 hours of inpatient stay (Figure 7). Although a lower percentage of doses were either delayed or omitted, the difference was not statistically significant (p>0.05).
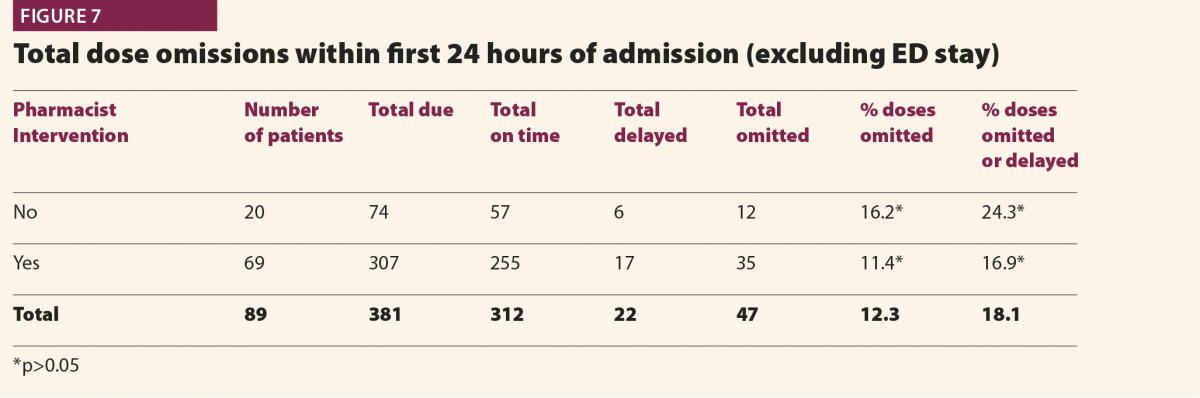
The mean length of stay for patients who did not receive pharmacist intervention in the ED was 10 days, which was similar to a mean of 11 days in patients who had a pharmacist intervention. However a trend was observed where generally longer inpatient stays were found in patients who had a higher percentage of missed doses in the first 24 hours of inpatient stay combined with their ED stay (Figure 8). This trend was not found to be statistically significant.
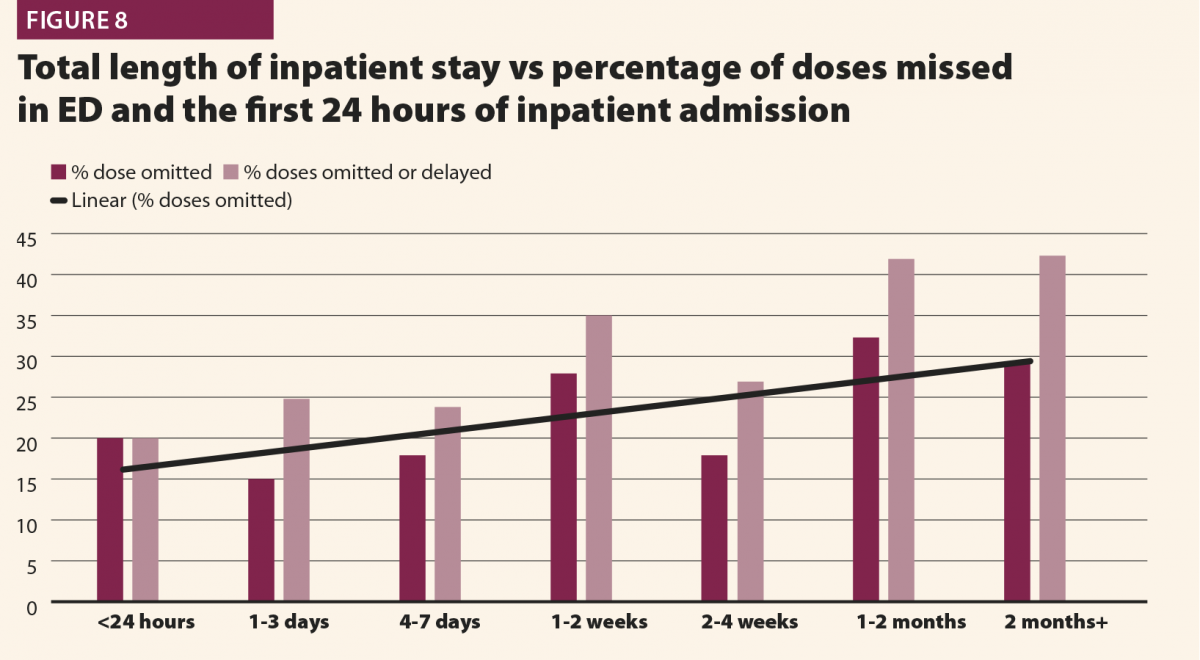
No particular trend was observed when total length of inpatient stay was plotted against the number of co-morbidities recorded for the patient (Figure 9). The relative numbers of co-morbidities patients suffered remained at a consistent percentage irrespective of total length of stay. Age may be associated with risk of prolonged stay (Figure 10), with only 10% of patients under the age of 70 spending more than seven days as an inpatient, compared with 53.3% over the age of 70.
All cases were also assessed for deterioration of clinical PD symptoms (as defined by increased stiffness, swallowing difficulties or reduced mobility from baseline) in the admitted patients. There is documented clinical worsening of PD symptoms for ten patients while in the ED. None of these cases involved a pharmacist intervention. In all ten cases, patients had at least one dose omitted in ED.
Medication prescribing errors were detected in a total of 16 cases where APMs were prescribed in the ED. These errors related to inappropriate dose, frequency or formulation of the medication. A further four of these cases that had pharmacist intervention had errors corrected immediately, with patients not receiving any incorrect doses.
The results demonstrate the potential impact of a consistent pharmacy presence on this high-risk population of patients. Furthermore, these findings lend weight to previous studies highlighting EDs as high-risk environments. Lastly, clear risk has been identified of patients deteriorating from baseline as a result of delayed medicines reconciliation within ED.
A total of 151 patients were identified and analysed who suffered from PD throughout the two-month study period. Of these, 89 (58.9%) required inpatient admission. This figure more than doubles the approximate average percentage internally of all patients who require inpatient treatment (26%). The average age of approximately 80, reflects a considerably older population of patients suffering from PD.
This finding is generally consistent with literature, considering patients suffering from PD typically are older, and are at higher risk of both medical and traumatic injuries.
A total of 62.6% APM doses were missed within the ED with no clinical reasoning, which demonstrates extremely poor compliance with internal guidance on continuation of time-critical medications, as well as national guidance.7 This figure is consistent with a similar previous study,17 which found a 74.6% dose omission rate
at another acute hospital Trust, indicating the problem may not be isolated to individual EDs and may be explained by systems failures. This may be due to compounding factors in the ED such as time pressure, overcrowding, lack of awareness of missed APMs and other priorities set by clinicians focusing on treatment of the presenting complaint – rather than maintenance of chronic therapies.
As previously discussed,18-20 APM omissions can be associated with potentially rapid and severe deterioration in symptoms. These are of particular concern in secondary care owing to development of dysphagia, potentially leading to aspiration, and mobility reduction increasing the likelihood of falls. Of the cases reviewed, clear documentation was found in ten cases (11.2%) where omissions may have lead to clinically significant deterioration of symptoms. These situations occurred exclusively in patients who did not have pharmacist review.
Decisions to admit may have been influenced by these deteriorations in conjunction with pathogenic disease, and caused extensions in inpatient stay as a result.
Pharmacist intervention occurred in 21.3% of the cases examined. This figure could be expected considering the level of service provision, which equates to 37.5 hours per working week, and that pharmacist responsibilities would have also extended to other high-risk patients. Pharmacists’ skill sets are well suited for this particular role, and would likely be superior to either a nurse- or medic-led approach to identifying and prioritising PD patients, due to innate knowledge of dosing schedules, formulation substitutions and rapid sourcing and supply.
Pharmacist intervention was associated with statistically significant reduction in dose omissions while in the ED and within the first 24 hours of inpatient admission. The effect within the ED can be explained by pharmacists identifying patients as high-risk at an early stage, having APMs prescribed and supplied and implementing treatment plans while in the ED. Thus, prescribing, supply and administration of APMs are all accelerated.
This approach has a completely different prioritisation strategy to typical ED processes.
The effect was not expected to be as profound at a ward level; however, data clearly demonstrate dramatic reductions in dose omissions after transfer from ED to base ward. This effect at base ward level is potentially explained by the improved handover and continuity of care that a pharmacist can provide in regards to medication supply and documentation of checked medication regimes. It was also noted from the results that a reduction in dose omissions (although not statistically significant) was observed in patients who had regular APMs prescribed within ED against those who did not. Patients might experience extended base ward waits prior to medical clerking which, when combined with omission in the ED, can lead to considerable proportions of time before normal medication can be reconciled and given.
It was, however, important to note that pharmacist intervention was unable to eliminate dose omissions. This is likely explained by pharmacists identifying patients once doses had already been missed in the department, and relying on nurse administration of medications by prompting rather than administering themselves.
These data indicate a tangible benefit in patient care and a clear prevention of deterioration within the department facilitated by early pharmacist intervention. These included two cases where patients attended the ED unable to resume oral therapies due to acute illness, where pharmacists rapidly recommended alternative measures to allow symptom control. Deterioration was not detected in these patients whilst in the department. These early deteriorations in PD symptoms which were excluded by previous studies may play important roles in preventing hospital admission, and as this study was not designed to assess this, further work might be required.
No conclusive effect was observed in regards to a reduction in inpatient stay in this study. A visual trend was observed where a higher percentage of dose omissions were observed in patients who were admitted for longer, though no statistical significance can be determined from these results.
Furthermore, no particular relationship was observed between more co-morbidities and increased length of stay. There was a noted potential relationship between increased age and longer length of stay, with a higher percentage of patients over the age of 70 requiring inpatient stays of over seven days.
A recent study18 found that a specialist PD unit within secondary care reduced APM omissions, increased timely administration and, importantly, demonstrated a reduced length of stay compared with other non-specialist ward areas. The specific effect of the number of dose omissions on the total length of stay was also explored in another hospital19 finding a significant increase in length of stay in patients who had a delay or missed at least one APM dose. These studies demonstrate potentially drastic implications on patient safety in secondary care, as well as financially in terms of reduced admissions lengths and flow through hospitals.
A recent larger scale study20 was conducted to determine the effect of delayed administration of APM on total length of stay. Ultimately the study did not demonstrate a statistically significant relationship between dose omissions and length of stay. Despite the lack of an association, the authors were keen to highlight that dose omissions were still detrimental to the best patient care. It was notable, however, that all studies included inpatient areas only and made no analysis of patient’s assessment and stay within EDs. A short retrospective audit17 examined APM omission and found 76% of patients experienced delays or omissions during attendances to the ED. An important and highly relevant discussion point from this study was raised around how poor medicines reconciliation early in the patient’s journey in ED has the potential to cause extended inpatient stays and poorer outcomes. Very little literature is available exploring this effect.
Prompt identification of patients suffering from PD and requiring continuation of their therapies was identified as a key limiting factor in preventing dose omissions. Compared with other time critical medications explored in our previous analysis,15 APMs have relatively few clinically justifiable acute contraindications to their use. This differs considerably from insulin or anticoagulants, for example, where the diagnostic process within the ED can require specialist clinical consideration and potentially be more clinically justifiable to temporarily suspend treatments.
This may partly explain how pharmacists might have such a high impact in these patients within the ED, considering the main adjustment to therapies made acutely were to convert formulations in light of swallowing difficulties or convert to dopamine agonist patches because of the clinical decision for a patient to be kept nil by mouth. Both of these interventions are typically regarded as requiring specialist clinical pharmacist input, which was not only readily available in this case, but actively provided and prioritised.
The methodology of this study has several limitations, the key being the relatively small sample size. This sample size does not provide enough data points to generate statistical power to prove a hypothesis, and thus this study was described as hypothesis generating.
Data gathering by free text keyword search may exclude a population of patients who had not been documented as having PD, and thus patients identified may be an underestimation. Furthermore, the methodology in this study assumed no documentation as an omission, whereas patients might have actually taken their own medication while in the department. Equally, when doses were signed administered, these were assumed to be given on time, where poor practice of not documenting the time administered might have changed these occasions into delays.
Information around the severity of the presenting complaint was not taken into account, because the spectrum of the acute presenting complaint might affect the ability of ED clinicians to prescribe drugs, such as in situations of life-threatening trauma or cardiac arrest. These situations may have more close correlations with dose omissions, rather than pharmacist intervention. Furthermore, situation of admission may also more significantly affect a total length of inpatient stay.
Pharmacist intervention was associated with a statistically lower number of omissions of APMs while in the ED. This intervention was associated with a lower incidence of PD-related deterioration in symptoms while in the department. Furthermore, as the interventions involved early medicines reconciliation, supply and handover of care, beneficial effects on dose omissions were observed on base ward level also.
These results also clearly demonstrate the potential deficiencies of EDs in reconciling and continuing chronic therapies in the acute setting. Readily available clinical pharmacists are an ideal choice in taking on this responsibility and taking pressure off ED clinicians, as well as yielding time and efficiency savings on base ward level.
The relatively small amount of data collected makes it difficult to comment on conclusions around an effect on length of patient stay. It is likely that a combination of factors such as age, co-morbidities and presenting complaint has impacts on length of stay and outcomes, and greater numbers of patients would need to be analysed to draw concrete conclusions.
Further study is required in exploring clinical pharmacy services within EDs on other high risk patient profiles, to determine if similar benefits can be identified.
Kunal Gohil MPharm PGDip PGCert
Obiageli Ngwuocha MBBS MRCEM
Rasheed Ashraf Ali FRCEM FRCS
1 Gerlach O et al. Deterioration of Parkinson’s disease during hospitization: Survey of 684 Patients. BMC Neurology 2012:12:13.
2 Guttman M et al. Parkinsonism in Ontario : Comorbidity associated with hospitalization in a large cohort. Mov Disord 2003;17:45–54.
3 Wood B et al. Incidence and prediction of falls in Parkinson’s disease : a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry 2001;72:721–5.
4 Haddad S et al. Prognostic factors associated with short-term decompensation of sepsis in the emergency department. Acad Emerg Med 2014;21(5).
5 Fernandez H, Lapane L. Predictors of mortality among nursing home residents with a diagnosis of Parkinson’s disease. Med Sci Monit 2002;8:241–6.
6 Parkinsons Disease Society. Emergency management of patients with Parkinsons. www.parkinsons.org.uk/professionals/resources/emergency-management-patie… (accessed December 2017).
7 National Patient Safety Association (NPSA). Reducing harm from omitted and delayed medication in hospital. www.nrls.npsa.nhs.uk alerts/?entryid45=66720 (accessed December 2017).
8 Sin B et al. Implementation of a 24-hour pharmacy service with prospective medication review in the emergency department. Hosp Pharm 2015;50(2):134–8.
9 Rothschild J et al. Medication errors recovered by emergency department pharmacists. Ann Emerg Med 2010;55(6):513–21.
10 Stasiak P et al. Detection and correction of prescription errors by an emergency department pharmacy service. CJEM 2014;16(3):193–206.
11 Marconi G, Claudius I. Impact of an emergency department pharmacy on medication omission and delay. Paediatric Emerg Care 2012;28(1):30–3.
12 Gohil.K. ED and Inpatient waiting times for higher risk medical patients. Internal Audit, Nottingham University Hospitals;2016.
13 Kaboli P et al. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med 2006;166(9):955–64.
14 Lord Carter of Coles. Operational productivity and performance in English NHS Acute Hospitals: unwarranted variations. www.gov.uk/government/uploads/system/uploads/attachment_data/file/499229… (accessed December 2017).
15 Gohil K, Patel C, Sani. M. Integrating a clinical pharmacy service in the ED. Hospital Pharmacy Europe 2016;83, accessed online 10/3/17 from http://www.hospitalpharmacyeurope.com/pharmacy -practice/integrating-clinical-pharmacy-service-ed
16 Henderson K, Gotel U, Hill J. Using a clinical pharmacist in the Emergency Department. EmergMed J 2015;32(12):RCEM Abstract 045.
17 Magadalinou K, Martin A, Kessel B. Prescribing medications in Parkinsons disease (PD) during acute admissions to a District General Hospital. Parkinsonism Relat Disord 2006;13:539–40.
18 Skelly R et al. Does a specialist unit improve outcomes for hospitalized patients with Parkinson’s Disease? Parkinsonism Relat Disord 2014;20:1242–7.
19 Martinez-Ramirez D et al. Missing dosages and neuroleptic usage may prolong length of stay in hospitalized Parkinson’s disease patients. PLoS ONE 2015;10:e0124356.
20 Skelly R, Brown L, Fogarty A. Delayed administration of dopaminergic drugs is not associated with prolonged length of stay in hospitalized patients with Parkinson’s Disease. Parkinsonism Relat Disord 2017;35:25–9.