This website is intended for healthcare professionals only.
Take a look at a selection of our recent media coverage:
25th November 2019
The work identifies PKM2 as a potential therapeutic target for treating a host of diseases mediated by over-active immune cells. The findings are reported in the journal Cell Metabolism – with the chief discovery being that PKM2 is a central ‘on’ switch for these cells.
Lead author Stefano Angiari, working with a team led by Luke O’Neill, Professor of Biochemistry in the School of Biochemistry and Immunology in the Trinity Biomedical Sciences Institute, has been exploring the role of PKM2 in the regulation of two cell types: Th17 and Th1.
Dr Stefano Angiari, Trinity, said: “Th17 and Th1 cells are very important for the damage that happens in autoimmune diseases such as psoriasis and multiple sclerosis. We have found that interfering with PKM2 blocks these cells and limits inflammation.”
Professor Luke O’Neill added: “PKM2 is a fascinating protein that has a role in how cells use glucose for energy, but it also moonlights in the immune system, where we have found it can be especially troublesome. We are currently exploring it as a new target for therapies that might work in patients with diseases like psoriasis and multiple sclerosis, where treatment options are limited.”
21st November 2019
The introduction of the digital sepsis alert system at Imperial College Healthcare NHS Trust in 2016 was associated with lower odds of death, shorter hospital stays and increased odds of receiving timely antibiotics.
The system monitors a range of changes in patients such as temperature, heart rate and glucose levels and alerts doctors and nurses if they fall outside safe parameters so they can investigate further. Clinicians are notified of patients who have triggered the alert either through a pop-up warning on their electronic health records and/or on a dashboard, which highlights any patient with an active alert when they open a patient’s record.
In addition to the alert, Imperial College Healthcare NHS Trust designed a multidisciplinary care plan which is launched in the electronic patient record system when a clinician confirms a diagnosis of sepsis. This prompts the clinical team to determine the best options from a range of treatments, such as fluids, oxygen, diagnostic tests and early antibiotics, and ensure they are given to patients within one hour – in line with national targets.
The study, led by researchers at Imperial College London and published in the Journal of the American Medical Informatics Association, is the first evaluation of a digital sepsis alert system in a British hospital trust and the largest undertaken anywhere to date.
Dr Kate Honeyford, from the Global Digital Health Unit at Imperial College London and lead author of the research, said: “Sepsis can be deadly if it’s not diagnosed and treated quickly. However, symptoms can be hard to spot and are similar to other conditions such as flu or a chest infection, which can result in delayed diagnosis and treatments. The sepsis alert system was put in place at Imperial College Healthcare NHS Trust to see if it can help monitor and flag patients who may have sepsis to clinicians for further investigations and treatments. Often digital systems are implemented but research on their performance is not done. Our study shows for the first time that robust analysis of a digital alert system was associated with improvements in outcomes for patients and the system presents an opportunity to improve care for patients who may have sepsis.”
Dr Ceire Costelloe, Director of Imperial’s Global Digital Health Unit and co-author of the study, said: “There is a drive towards creating innovative digital technology that will meet the needs of patients and clinicians in the NHS. Our study shows that the sepsis alert system could be an example of this. The results are encouraging and we will be carrying out further work to see if the alert system has the same impact on a wider group of patients.”
Dr Anne Kinderlerer, Consultant Rheumatologist at Imperial College Healthcare NHS Trust and co-author of the study, added: “At Imperial College Healthcare NHS Trust we are focused on improving our ability to recognise and treat sepsis more quickly and effectively. The findings from this study show that the alert has made a significant impact on identifying more cases of sepsis and reducing the number of patients who die in hospital as a result. More patients are surviving sepsis at our hospitals and it is testament to the alert and treatment plans we have working hand in hand to help us ensure that patients are treated with antibiotics and other interventions in order to save more lives. Our plan is now to roll this alert system out across the Trust in different health specialities so that we can further reduce the toll and impact that sepsis has on our patients.”
The digital sepsis alert system was developed by Cerner and it was introduced at Imperial College Healthcare NHS Trust’s hospitals in 2016 within emergency departments and inpatient wards.
The team analysed data of more than 27,000 hospital stays of patients who had triggered the alert system between October 2016 and May 2018. These patients were in emergency departments as well as acute and haematology wards at St Mary’s Hospital, Charing Cross Hospital and Hammersmith Hospital, part of Imperial College Healthcare NHS Trust.
The team found that patients who triggered the alert had 24% lower odds of in hospital death as well as a 35% increased chance of receiving timely antibiotics than a group of patients who were receiving the usual standard care, where the digital alert system is not involved. They also found that patients who were admitted to hospital had a 4% lower chance of staying in hospital for more than seven days than patients with similar symptoms, than the group of patients who were receiving standard care.
The team suggests the alert system has been able to alert clinicians to deteriorating conditions in patients and, as a result, investigations and treatment plans have been implemented more quickly.
The team will now carry out a larger study involving more NHS hospitals to see whether the results are the same in a bigger patient group.
The European Commission has granted marketing authorisation for daratumumab in combination with lenalidomide and dexamethasone (Rd) for the treatment of newly diagnosed multiple myeloma patients who are ineligible for autologous stem cell transplant (ASCT).
The approval was based on results from the Phase III MAIA (MMY3008) study.
The study included 737 newly diagnosed patients with multiple myeloma ineligible for high-dose chemotherapy and ASCT aged 45-90 years old (median age of 73 years).
Daratumumab in combination with Rd significantly reduced the risk of disease progression or death by 44% in patients with newly diagnosed multiple myeloma who are transplant ineligible, compared to treatment with Rd alone (hazard ratio [HR] = 0.56; 95% confidence interval [CI]: 0.43-0.73; p<0.0001). At median follow-up of 28.0 months the median progression-free survival (PFS) for daratumumab-Rd had not yet been reached, compared to 31.9 months for patients who received Rd alone.
The addition of daratumumab resulted in deeper responses compared to Rd alone, including increased rates of complete response (CR) or better (48% vs 25%) and improved rates of very good partial response (VGPR) or better (79% vs 53%).1 Daratumumab-Rd induced a >3-fold higher rate of minimal residual disease (MRD) negativity compared to those who received Rd alone (24% vs. 7%).
“Despite recent therapeutic advances, relapse of multiple myeloma is considered to be almost inevitable, becoming more challenging to treat following each relapse. This makes it even more important that we maximise our best response upfront to extend the first remission,” said Professor Thierry Facon, MD, Service des Maladies du Sang, Hôspital Claude Huriez, Lille, France, and principal investigator of the MAIA study.
“This marks an important approval, especially for transplant ineligible patients, a more vulnerable population, for whom outcomes are generally poorer when compared to those who are transplant eligible.”
Dr Patrick Laroche, Haematology Therapy Area Lead, Europe, Middle East and Africa (EMEA), Janssen-Cilag, commented: “Every year over 48,000 people in Europe are diagnosed with multiple myeloma, which is considered to be incurable. Older patients who are ineligible for transplant have a limited range of frontline therapeutic options available, so we are pleased that with today’s approval of daratumumab-Rd, these patients now have a new frontline option available to them.”
Craig Tendler, MD, Vice President, Clinical Development and Global Medical Affairs, Oncology, Janssen Research & Development, LLC., added: “It’s gratifying to see that through our research and development efforts, daratumumab has helped over 100,000 patients globally. With today’s approval and the continued development of daratumumab, we hope to bring this innovative therapy to many more patients in the future.”
The initiative aims to tackle HIV-related health challenges faced by people living with HIV (PLWHIV), such as associated mental and physical co-morbidities, mental health issues, financial stresses and potential HIV-related stigma.
Recommendations in the white paper, which have been summarised in AIDS Reviews, focus on a new HIV care framework – Health Goals for Me. The recommendations were developed to support an holistic and individualised long-term treatment approach that goes beyond viral load and CD4 count, to assess health-related quality of life.
This international, expert alignment represents a pathway to achieving the ‘fourth 90’, a goal that builds on the United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 treatment target. Through Health Goals for Me, the Moving Fourth Expert Committee aims to increase focus on taking an individualised long-term approach to patient care, which forms the basis of our continued work to develop practical tools which can help improve health-related quality of life for PLWHIV.
The UNAIDS proposed the ambitious 90-90-90 treatment plan in 2014 to end the AIDS epidemic: to diagnose 90% of all HIV-positive persons; provide antiretroviral therapy (ART) to 90% of those diagnosed; and to achieve viral suppression for 90% of those treated by 2020. These targets, however, do not cover other HIV-related health challenges faced by people living with HIV.
“It is a pivotal moment in HIV,” said Dr Giovanni Guaraldi, Chair of the Moving Fourth Expert Committee and Associate Professor of Infectious Disease and Head of the Modena HIV Metabolic Clinic (MHMC), Italy, in launching the group’s first paper. “Pioneering research means the disease is now viewed as a chronic condition. However, this brings new challenges relating to long-term health; as increasing numbers of people navigate their lives beyond viral suppression and look towards improved quality of life. Health Goals for Me should become an intrinsic part of HIV care in the future.”
Mario Cascio, Former-Chair of European AIDS Treatment Group (EATG), Italy, who lives with HIV and is a member of the expert committee said: “Treatment guidelines and policy for HIV are much the same as when it was a fatal disease. Despite HIV being considered a chronic condition, many people are suffering with the day-to-day impact of the disease, such as continued stigma, discrimination and health conditions experienced after many years of living with HIV. This international consensus represents an important first step to improve care beyond viral load and ultimately improve the lives of people living with HIV.”
“The Moving Fourth steering committee was convened to establish a way in which the ‘fourth 90’ can be achieved,” said Allitia diBernardo, Europe, Middle East and Africa Infectious Diseases and Vaccines Therapeutic Area Lead, Janssen-Cilag International NV. “The ultimate aim of this initiative is to provide clear, detailed guidelines and tools that support a holistic, individualised, long-term treatment approach for PLWHIV that goes beyond viral load and CD4 count. At Janssen we recognise that, despite progress made, HIV remains one of the greatest global health threats of our time. We won’t stop until we end HIV.”
The Moving Fourth steering committee members are:
• Dr Giovanni GUARALDI (Chair), Associate Professor of Infectious Disease and Head of the Modena HIV Metabolic Clinic (MHMC), Italy
• Dr Joop ARENDS, Internist, Associate Professor of Infectious Disease and Infectious Diseases Physician, Department of Internal Medicine and Infectious Diseases, University Medical Centre Utrecht, The Netherlands
• Dr Thomas BUHK, Internist, Infectiologist, Centers for Infectious Diseases in Hamburg (ICH-Hamburg), Germany
• Mario CASCIO Former-Chair, European AIDS Treatment Group (EATG), Italy
• Dr Adrian CURRAN, Internist, Infectious Disease Specialist, Department of Infectious Diseases, Vall d’Hebron University Hospital, Barcelona, Spain
• Dr Eugenio TEOFILO, Internist, Department of Internal Medicine at Hospital Dos Capuchos, Lisboa, Portugal
• Dr Guido VAN DEN BERK, Internist, Infectious Disease Specialist, Department of Internal Medicine, OLVG, City Hospital of Greater Amsterdam, The Netherlands
• Christian VERGER, Chair of the Grand Est region, Board member, AIDES-France, France
If approved, Mayzent will be the first and only oral disease-modifying therapy for this patient population.
The CHMP positive opinion for Mayzent is based on data from the Phase III EXPAND study, a randomised, double-blind, placebo-controlled trial comparing the efficacy and safety of Mayzent versus placebo in 1651 people with SPMS with varying levels of disability (Expanded Disability Status Scale [EDSS] scores of 3.0 to 6.5). Mayzent was shown to reduce the risk of disability progression with a safety profile that was overall consistent with the known effects of S1P receptor modulation. Specifically, in the post hoc analysis of the active SPMS subgroup (n=779), results demonstrated that Mayzent reduced the risk of three- and six-month confirmed disability progression by 31% (p=0.0094) and 37% (p=0.0040) respectively versus placebo.
Additionally, a sub-group analysis of the EXPAND study showed that Mayzent had functional benefits on cognitive processing speed, the cognitive function most frequently affected by MS. Further analyses have shown that Mayzent may help patients preserve their mobility for over four years longer on average, and that it reduced loss of cortical grey matter and thalamic volume – key drivers of disability progression and declining cognitive function in patients with SPMS.
“Until recently, although we have made considerable strides in reducing relapses and long term disability in patients with relapsing remitting disease, there was little to offer patients who had reached the secondary progressive stage of the disease. Results from the EXPAND study demonstrate that Mayzent has a positive impact on reducing both cognitive and physical decline, offering renewed hope for people living with active SPMS,” said Dr Eli Silber, Consultant Neurologist at King’s College Hospital NHS Foundation Trust. “The CHMP’s positive recommendation heralds a new era of care for people with active SPMS, potentially offering the multiple sclerosis community, for the first time, a much-needed treatment that is proven to delay disability progression.”
“At Novartis, we are committed to reimagining care for patients across the MS spectrum, and today’s positive decision marks a significant step towards building a better future for people living with active SPMS,” said Haseeb Ahmad, Managing Director UK, Ireland and Nordics, Novartis Pharmaceuticals & Country President UK. “Mayzent offers the potential to expand possibilities for those living with the condition by delaying the progression of disability. We will continue working closely with regulatory bodies to make sure this new treatment is made available in the UK for those who it could benefit.”
Professor Matthias Löhr explains why increased awareness of pancreatic cancer and increased investment into the field should become an urgent priority across Europe.
On 21 November 2019, United European Gastroenterology (UEG) and Pancreatic Cancer Europe (PCE) recognises World Pancreatic Cancer Day, a global initiative developed to raise awareness and prompt action against one of the world’s deadliest cancers.
Over the past 50 years, diagnosis and treatment strategies for cancer patients have evolved rapidly, transforming patient outcomes. Despite the major advancements witnessed in other areas of oncology, improvements in pancreatic cancer patient outcomes have largely stood still. In sharp contrast to the remarkable growth in survival rates observed in other disease areas such as lung, breast or prostate cancer, the overall five-year survival rate for patients diagnosed with pancreatic cancer is just 5% across the globe, a figure that has not significantly improved since the 1970s.1
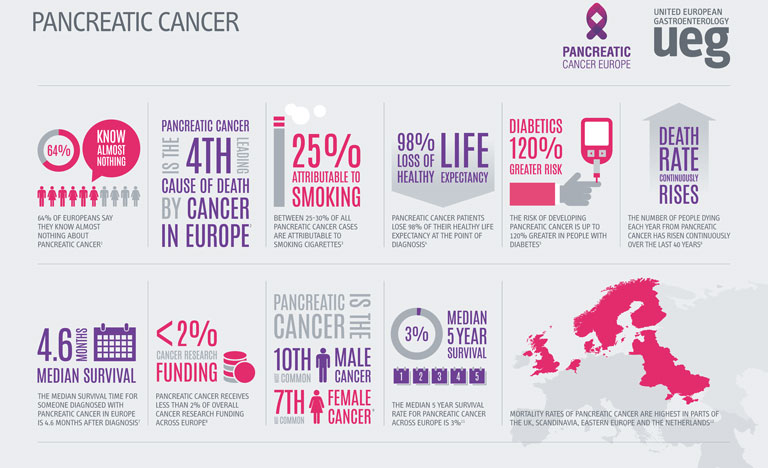
Compounding the threat of these concerning statistics, the incidence and mortality rates related to pancreatic cancer are on the rise globally. A recent study presented at UEG Week Barcelona 2019 revealed that as well as an increase in pancreatic cancer cases, the number of deaths attributable to the disease has risen from 196,000 in 1990 to 448,000 in 2017.1
Whilst a proportion of this increase can be explained by a rising population and increased life-expectancy, age-standardised incidence and death rates for pancreatic cancer had still risen by 12% and 10% respectively over the course of the study.1 Particularly of note, the highest incidence and death rates were recorded in higher-income countries.
Whilst the precise aetiology of pancreatic cancer remains unknown, a number of factors have been linked to the development of the disease. Obesity, an epidemic that has a close association with high-income countries, has been shown to increase a patient’s risk of pancreatic cancer by almost 47%.2 The ever-increasing prevalence of obesity and diabetes across the globe, coupled with an ageing population is set to add weight to the already-heavy burden of pancreatic cancer.
Recent forecasts have predicted that both the number of cases and deaths will increase by 40% by 2035 if preventative measures are not taken.3 With an estimated two-thirds of the risk factors being categorised as potentially modifiable, there is a huge opportunity for both clinicians and the public to promote and partake in lifestyle changes that can significantly reduce the risk of the disease.4
However, a number of pressing challenges still face clinicians and the general public in the identification and treatment of pancreatic cancer. In the earliest stages of the disease, symptoms are often silent or general, making pancreatic cancer a notoriously difficult disease to diagnose.
Perpetuating this problem further, poor public awareness of pancreatic cancer and the absence of a standard diagnostic tool frequently causes delays in identification, allowing the cancer to remain present in the body for many years prior to detection. As a result, only 10-20% of cases are identified in time for curative surgery.5 For those who are not diagnosed in time for resection, violent tumours can persist, which display extreme resistance to treatment, partially explaining the incredibly low survival rates associated with pancreatic cancer.
Despite these daunting statistics, recent progress has provided renewed hope for the future status of pancreatic cancer. Building upon a wealth of established research, a number of incremental improvements have been seen across the field, including the increased efficacy of a range of treatment options.
Traditionally, surgery, chemotherapy and radiation therapy have been the most commonly used tools in the fight against pancreatic cancer. However, the advent of immunotherapy could signify a new frontier for pancreatic cancer treatment.
Recent studies have suggested that combination treatment strategies in conjunction with immunotherapy could potentially yield positive results, improving pancreatic cancer prognosis.6 In order to provide robust and conclusive evidence of the benefits of immunotherapy, a closer examination of this treatment appears to be a worthwhile future endeavour.
In light of the clear need for further research into pancreatic cancer, the lack of funding provided to this area is particularly unsettling. European funding for the disease lies far behind many other cancers with similar mortality rates, receiving less than 2% of all cancer research funding in Europe.7 An increased allocation of EU funds to pancreatic cancer research will allow for the exploration of a number of different treatment options that could significantly improve patient outcomes across Europe.
The Cancer Moonshot programme, a project launched across the US with the aim of reducing mortality rates in several major cancers, represents a promising new development in pancreatic cancer research. The resultant Precision PromiseSM, an adaptive randomised clinical trial platform, allows researchers to evaluate multiple novel therapies to develop effective and ground breaking treatment options for pancreatic cancer.8 The implementation of similar projects across Europe and the wider world should be seen as an essential step in improving pancreatic cancer outcomes across Europe.
Solving the persistently difficult question of how to improve pancreatic cancer care will ultimately require not only the allocation of funds to the field, but a coordinated approach from all areas of society, including the public, academia and industry. Improving awareness of the disease amongst the public, encouraging researchers to enter the field and apply for grants, and incentivising drug companies to focus on drug development, are all crucial steps we need to take if we are to change the course of this destructive and often overlooked disease.
Matthias Löhr MD PhD
Professor of gastroenterology and hepatology, Karolinska Institutet, Sweden, and member of the UEG Public Affairs Committee
19th November 2019
A new guideline on the treatment of drug-resistant tuberculosis (DR-TB) has been published.
The guideline from The American Thoracic Society, Centers for Disease Control and Prevention, European Respiratory Society and the Infectious Diseases Society of America makes new recommendations for the choice and number of drugs, as well as the duration of treatment for DR-TB. These recommendations prioritise the use of medications that can be administered orally.
The guideline makes clear that treatment should be tailored based on drug-susceptibility testing, and that individuals should not receive medicines to which the Mycobacterium tuberculosis strain is resistant.
The guideline includes two other new recommendations. It recommends treatment with a later-generation fluoroquinolone of all infected contacts of multidrug-resistant tuberculosis (MDR-TB) patients, rather than watchful observation, and it provides evidence-based guidance for the treatment of pregnant women with MDR-TB for the first time.
Treatment for MDR- and extensively drug-resistant (XDR)-TB is long and difficult. The availability of potent new and repurposed medicines allows practitioners, for the first time, to choose alternatives to injectable drugs, which have long been considered an essential component of treatment regimens for DR-TB. These injectable drugs have well-known toxicities, including irreversible hearing loss.
By prioritising the use of orally administered medicines, the guideline writing committee believes clinicians can spare patients some of the most debilitating effects of TB treatment, make treatment more tolerable and improve outcomes.
The guideline committee included specialists in pulmonary medicine, infectious diseases, paediatrics, primary care, public health, epidemiology, economics, pharmacokinetics, microbiology, systematic review methodology and advocacy.
“Having the participation of committee members from multiple medical societies and the CDC, as well as patient advocate perspectives, was absolutely critical to discussing the balance between desirable and undesirable health effects of interventions, making certain they were favourable for MDR-TB patients, including for children and pregnant women,” said Payam Nahid, MD, MPH, chair of the guideline committee and a professor of medicine at the University of California, San Francisco.
This guideline is generally consistent with the World Health Organization’s recommendations but includes some different recommendations that are specifically tailored to low-incidence, higher-resource countries of North America and Europe.
In making its recommendations, the committee reviewed findings from 50 studies, conducted in 25 countries involving more than 12,000 patients with DR-TB.
The committee grouped questions to be answered by the guideline into six topics:
1) number of effective drugs in a regimen for DR-TB, duration of intensive and continuation phases of treatment for DR-TB;
2) review of drug and drug classes for the treatment of DR-TB;
3) the use of a standardised, shorter-course regimen of < 12 months for the treatment of MDR-TB;
4) treatment of isoniazid-resistant, rifampin-susceptible TB;
5) surgery as adjunctive therapy for MDR-TB; and
6) treatment of contacts of persons with MDR-TB.
The committee used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework to indicate the strength of the evidence supporting the 25 recommendations included in the clinical guidance.
“The individual patient data level analyses conducted by investigators at McGill University were essential to the development of our recommendations, representing a substantial analytic advance over prior approaches that relied on aggregate data,” said Barbara Seaworth, MD, guideline committee co-chair and medical director of the Heartland National TB Center, at the University of Texas Health Science Center at Tyler. “However, the certainty in the evidence for observational studies is lower than for clinical trials, and much greater investment is urgently needed to allow the TB research community to conduct high-quality randomised, interventional TB clinical trials that will define safe and effective all-oral regimens for treatment of all patients with DR-TB.”
18th November 2019
Researchers say their test, which works by detecting chemical clues shed by brain tumours into the blood, could help improve brain tumour survival by making diagnosis quicker and more efficient.
Dr Paul Brennan, senior clinical lecturer and honorary consultant neurosurgeon at the University of Edinburgh, UK, said: “Brain tumours reduce life expectancy by an average of 20 years. That’s the highest of any cancer.
“We know that 62% of patients are diagnosed in the emergency department, even though they may have seen their GP several times beforehand. This is because diagnosing brain tumours is so difficult. A headache could be a sign of a brain tumour, but it is more likely to be something else and it’s not practical to send lots of people for a brain scan, just in case it’s a tumour. The challenge is identifying who to prioritise for an urgent scan.”
Dr Brennan has worked with Dr Matthew Baker, reader in chemistry at the University of Strathclyde, UK, and chief scientific officer at ClinSpec Diagnostics Ltd to develop a test to help doctors to quickly and efficiently find those patients who are most likely to have a brain tumour.
The test relies on infrared spectroscopy to examine the chemical makeup of a person’s blood, combined with an AI program that can spot the chemical clues that indicates the likelihood of a brain tumour.
The researchers tried out the new test on blood samples taken from 400 patients with possible signs of brain tumour who had been referred for a brain scan at the Western General Hospital in Edinburgh, UK. Of these, 40 were subsequently found to have a brain tumour.
Using the test, the researchers were able to correctly identify 82% of brain tumours. The test was also able to correctly identify 84% of people who did not have brain tumours, meaning it had a low rate of ‘false positives.
In the case of the most common form of brain tumour, called glioma, the test was 92% accurate at picking up which people had tumours.
Dr Baker said: “These results are extremely promising because they suggest that our technique can accurately spot who is most likely to have a brain tumour and who probably does not.
“Because the technique requires just a small blood sample, if offers the potential to test a large number of people with suspicious symptoms and give the best indication of who needs an urgent brain scan. This could ultimately speed up diagnosis, reduce the anxiety of waiting for tests and get patients treated as quickly as possible.”
The next step will be to try out the test with 600 more patients who have either been referred for a brain scan via their GP or the hospital emergency department. The researchers say a much smaller proportion of these patients will be subsequently diagnosed with a tumour.
The researchers also say the same technique has the potential to be adapted to other types of cancer that are difficult to diagnose, such as ovarian, pancreatic, bowel and prostate cancer.
Dr Sarah Jefferies, is a member of the NCRI’s glioma subgroup and Clinical Director for Cancer at Addenbrooke’s Hospital, Cambridge, UK and was not involved in the research. She said: “The number of people being diagnosed and dying from brain tumours is increasing and we urgently need better ways to spot and treat the disease.
“This type of testing offers a number of potential advantages. It’s relatively straight-forward for the patient, who need only have a blood test. For the health service, it could combine with clinical assessment to make the process of referring patients for brain scans more efficient.
“We look forward to further assessment of this technique.”
14th November 2019
The study, published in Nature Cell Biology, looked into the most common type of pancreatic cancer, pancreatic ductal adenocarcinoma. This is an aggressive cancer that develops from secretory and tubular cells of the pancreas.
There are no effective therapies to treat this cancer and only 8% of patients survive beyond five years after diagnosis.
The researchers analysed cancer stem cells. Similar to how healthy human stem cells repair tissues and organs, these cells have the ability to start new tumours and they can also differentiate into different types of tumour cells.
As these cells are a driving force behind cancer growth, being able to identify if they are present is an important step towards the development of new treatments. By analysing the gene expression of these cancer stem cells, the team found that a protein, called CD9, is present on their surface both when the tumour is developing and when it is more established. This protein could therefore be used as a marker to help locate these cells.
The study further established that this protein is not just a marker of cancer stem cells, but also promotes their malignant behaviour. The researchers altered the amount of CD9 in tumour cells in mice and found that when the levels of this protein were reduced, smaller tumours formed. Conversely, increasing levels of CD9 made cancer cells more aggressive and able to form large tumours quickly.
These findings were supported by existing clinical data showing that patients whose tumour cells have more CD9 have a poorer clinical prognosis. About 10% of people with this type of cancer have amplified levels of CD9.
“These cells are vital to pancreatic cancer and if even just a few of them survive chemotherapy, the cancer is able to bounce back. We need to find effective ways to remove these cells, and so stop them from fuelling cancer growth. However, we need more experiments to validate the importance of CD9 in human pancreatic cancer,” says Victoria Wang, lead author and member of the Adult Stem Cell Laboratory at the Crick.
To understand the mechanism behind how CD9 bolsters cancer, the team looked into the cancer stem cells’ metabolism. Their findings showed that CD9 increases the rate cells take up glutamine.
“Now we know this protein is both linked to cancer stem cells and helps cancer growth, this could guide the development of new treatments that are targeted at the protein and so cut off the supply of glutamine to cancer stem cells, effectively starving the cancer,” says Axel Behrens, corresponding author and group leader in the Adult Stem Cell Laboratory at the Crick.
NICE has recommended that elmiron®, the only licensed oral medication for BPS with glomerulations or Hunner’s lesions (referred to as interstitial cystitis/BPS), is used in accordance with specified considerations.
The recommendation came after Consilient Health agreed a Patient Access Scheme with NICE which makes elmiron® available to the National Health Service (NHS).
Mr Jonathan Goddard, Consultant Urological Surgeon, Leicester General Hospital said: “This positive recommendation from NICE will allow patients with this chronic and difficult to treat condition to benefit from elmiron®, an effective and well-tolerated treatment for BPS”.
Susannah Fraser, Communication and Media Manager, Bladder Health UK said: “BPS is a debilitating condition and has a considerable detrimental impact on the individual’s lifestyle, ability to work, emotional health and relationships. Their quality of life is hugely compromised. For individuals to be able to access elmiron® on the NHS is really good news.”
Amanda Paxon, Consilient Health UK Country Manager Pharmaceuticals said: “This is a condition with a significant unmet clinical need and the company is delighted that elmiron® has been recommended for the treatment of IC/BPS”.