This website is intended for healthcare professionals only.
Take a look at a selection of our recent media coverage:
30th May 2025
A study has revealed that centralised body fat is more strongly linked to an increased risk of psoriasis than overall body fat.
Researchers led by King’s College London found that the location of body fat, rather than just overall body mass, plays a critical role in the likelihood of developing psoriasis. Women with high levels of abdominal fat were most at risk.
The researchers hope that the findings, published in the Journal of Investigative Dermatology, could help to improve early risk prediction and guide personalised prevention strategies for psoriasis.
As global obesity rates continue to rise, the importance of understanding how different patterns of body fat influence inflammatory diseases such as psoriasis becomes increasingly significant.
Using data from the UK Biobank, the researchers studied 336,806 participants of White British ancestry, including 9,305 individuals with a diagnosis of psoriasis. Some 25 different measurements of fat were taken, including body measurements and advanced imaging techniques, to differentiate between types of fat, such as visceral and subcutaneous fat, and to record the precise locations of fat within the body.
The researchers found that people with more fat around their waist and abdomen are more likely to have psoriasis. Out of the five measures most strongly associated with the development of psoriasis, four were measures of central body fat. These include the waist-to-hip ratio, abdominal fat ratio, total abdominal adipose tissue index and waist circumference.
Among the various methods for measuring total adiposity, the researchers found that the percentage of body fat, as measured using bioelectrical impedance analysis (BIA), had the strongest association with psoriasis, indicating that higher overall body fat increases the likelihood of developing psoriasis.
Professor Michael Simpson, professor of genetics at King’s and co-author of the study, added: ‘Previous work has shown that people with psoriasis who carry a particular gene variant, called HLA-C06:02, tend to have lower overall body fat. However, our analysis demonstrates that abdominal fat increases psoriasis risk regardless of whether or not people have this gene variant. This suggests that central body fat is a causal risk factor for psoriasis, no matter your genetic background.’
Future studies will examine a broader cohort of ethnicities and aim to validate the findings and enhance risk prediction across diverse groups.
This article was originally published by our sister publication Nursing in Practice.
Additional scans on top of mammography can detect more cases of breast cancer when offered to women with very dense breast tissue, a UK trial has reported.
A trial of more than 9,000 women at 10 sites found that extra imaging done with abbreviated MRI or contrast-enhanced mammography picked up cancers that had not been detected with standard mammogram.
If the approach was adopted on the NHS, the extra scans could treble cancer detection potentially saving up to 700 lives a year in the UK, the researchers at the University of Cambridge reported in The Lancet.
Around 10% of women women have very dense breasts as shown by mammogram. Between the ages of 50 and 70, they are up to four-times more likely to develop breast cancer compared to women with low breast density.
Per 1,000 women screened, the two scanning methods detected 17-19 cancers that were not seen in mammograms. Adding either of them to existing screening programmes could detect 3,500 more cancers a year, the team calculated.
A third method – automated whole breast ultrasound – did pick up more cases than mammography alone but was not as effective as abbreviated MRI or contrast-enhanced mammography.
It is the first trial to directly compare supplemental imaging methods and to investigate their value for early cancer detection as part of widespread screening.
But further research is needed to confirm whether picking up extra cases of cancer through the additional scans leads to a reduction in the number of deaths.
Study lead Professor Fiona Gilbert, honorary consultant radiologist at Addenbrooke’s Hospital in Cambridge, said: ‘Getting a cancer diagnosis early makes a huge difference for patients in terms of their treatment and outlook.
‘We need to change our national screening programme so we can make sure more cancers are diagnosed early, giving many more women a much better chance of survival.’
Professor Stephen Duffy, emeritus professor at Queen Mary University, London, trial statistician said: ‘The NHS Breast Screening Programme has made a huge difference to many lives. Thanks to these results we can see that the technology exists to make screening even better, particularly for the 10% of women with dense breast tissue.’
Dr David Crosby, head of prevention and early detection at Cancer Research UK, said: ‘This study shows that making blood vessels more visible during mammograms could make it much easier for doctors to spot signs of cancer in women with dense breasts.
‘More research is needed to fully understand the effectiveness of these techniques, but these results are encouraging.’
This article was originally published by our sister publication Pulse.
12th March 2025
Research has uncovered clear differences in the development of hypertension between South Asian and East Asian adults.
The findings from 3,400 adults enrolled in UK Biobank suggest South Asian and East Asian adults living in the country may have distinct trajectories to developing hypertension as well as outcomes.
Data on patients with at least two blood pressure measurements recorded in their GP notes, as well as information on future heart disease events, including heart attacks, stroke and peripheral artery disease from hospitalisation and outpatient care records, was used to develop a model to assess patterns of blood pressure and how that related to cardiovascular disease risk.
It showed that South Asian adults living in the UK underwent earlier and faster increases in blood pressure compared to East Asian adults.
On average, South Asian men were also projected to reach a systolic blood pressure of 130 mmHg or higher 10 years sooner than East Asian men, at 36 years of age compared with 46 years, respectively.
In women, the gap was seven years (45 vs 52 years), the researchers reported in the journal Hypertension.
For South Asian adults, high blood pressure seen in early adulthood was associated with higher lifetime cardiovascular disease risks.
By contrast in East Asian adults, higher blood pressure in midlife was associated with higher atherosclerotic cardiovascular disease risk; even at ages 65 and older, high blood pressure was associated with heightened risk of stroke, the researchers said.
Systolic blood pressure in East Asian adults ages 65 years or older was significantly linked to all types of stroke risk.
And young adulthood diastolic blood pressure was strongly linked to peripheral artery disease in South Asian adults with a 2.18 times higher risk per standard deviation increase.
The team also found that South Asian adults had higher blood pressure readings and were at least three times more likely to be on antihypertensive medications compared to East Asian adults.
Within UK Biobank, participants who self-identified as originating from India, Pakistan, Bangladesh, Bhutan, Maldives, Nepal or Sri Lanka are defined as South Asian and those who self-identified as originating from China are defined as East Asian.
Study author Dr Pradeep Natarajan, an associate professor at Harvard Medical School, said: ‘These findings demonstrate the need to tailor blood pressure screenings and treatment timing for different Asian subpopulations to advance personalised care and prevention strategies for historically understudied communities.
‘Distinct age-related blood pressure patterns provide valuable insights to better manage cardiovascular risks and improve care for diverse populations.’
Dr Nilay Shah, a cardiologist who was chair of the American Heart Association’s position statement on cardiovascular health in Asian Americans said the paper highlighted important evidence to support the notion that cardiovascular risk factors like hypertension are not uniformly experienced among the diverse communities that are frequently labelled under the label ‘Asian’.
The findings should also prompt greater exploration of differences in social risk factors that may explain the differences in hypertension and CVD outcomes among self-reported Asian ethnicity groups, he added.
‘Ultimately, these findings from a UK population of Asian adults suggest a complex interplay of social factors and genetics resulting in varying experiences of hypertension in Asian populations.
‘There is much, much more work to be done to understand cardiovascular risk factors and outcomes experienced by Asian populations.’
Previous research has shown that clopidogrel is ineffective for myocardial infarction prevention in majority of British South Asians.
A version of this article was originally published by our sister publication Pulse.
10th March 2025
An artificial intelligence tool (AI) that predicts a patient’s risk of falls is being rolled out nationally by the NHS to increase efficiency and reduce hospital admissions.
The tool, developed by health tech provider Cera, is now being used in two million patient home care visits a month, across two thirds of integrated care systems. It monitors vital health signs to predict signs of deterioration and alerts healthcare staff so they can intervene and reduce the risk of hospitalisation.
NHS England hopes the tool will prevent around 2,000 falls and hospital admissions each day.
Falls are currently the largest cause of emergency hospital admissions, with around 30% of those above 65 years of age and half of those over 80 experiencing a fall at least once a year, according to NHS England.
The tool is also set to be used to detect the symptoms of illnesses such as Covid-19, flu, respiratory syncytial virus (RSV) and norovirus, with the aim of reducing hospital admissions for these conditions.
Dr Vin Diwakar, national director of transformation at NHS England said: ‘This new tool now being used across the country shows how the NHS is harnessing the latest technology, including AI, to not only improve the care patients receive but also to boost efficiency across the NHS by cutting unnecessary admissions and freeing up beds ahead of next winter, helping hospitals to mitigate typical seasonal pressures.
‘We know falls are the leading cause of hospital admissions in older people, causing untold suffering, affecting millions each year and costing the NHS around £2bn, so this new software has the potential to be a real game-changer in the way we can predict, prevent and treat people in the community.’
Responding to the news, Matthew Taylor, chief executive of the NHS Confederation, welcomed the rollout but warned that technology should be ‘robustly evaluated’ when used for patient care.
He said: ‘The rollout of this new artificial intelligence tool is a very welcome move that can reduce falls and hospital admissions – so both preventing harm to patients and saving money. It is yet another example of the exciting opportunities that AI offers the healthcare sector.
‘But with many innovative uses of AI rapidly developing, it’s important that while we keep an open mind to these opportunities we also adhere to frameworks and guidance to ensure any use of technology is robustly evaluated for patient care, level of productivity and overall impact on the NHS.
‘There is a lot of untapped potential but much of it is not fully understood. We need to make sure we are using tools that solve genuine problems NHS organisations or staff are facing.‘
Investment in these technologies is ‘crucial‘ to supporting ‘the Government’s ambitions of moving from an analogue system to one that is fully digitised’, he added, emphasising that ’the NHS needs to be supported to improve the digital infrastructure that underpins the latest technologies’.
Last year, the NHS began using AI to identify patients likely to become frequent users of emergency departments across England so it can be proactive about their care and reduce demand on these services.
The Government set out a new AI action plan earlier this year, to try and help the public sector spend less time on admin and more on delivering services. While health secretary Wes Streeting has previously said that shifting from analogue to digital is the priority within this parliament.
A version of this article was originally published by our sister publication Healthcare Leader.
1st November 2024
Artificial intelligence (AI)-enabled electrocardiography (ECG) can accurately predict an individual patient’s risk of future cardiovascular events as well as their short and long-term risk of dying, a study finds.
Existing AI-enabled ECG could predict disease and mortality but could not give sufficient information to guide clinical decisions for individual patients and were not adopted into practice, UK researchers wrote in The Lancet Digital Health.
To address the limitations of previous AI models, the team from Imperial College London and Imperial College Healthcare NHS Trust developed the AI-ECG risk estimator (AIRE) platform using data from a secondary care dataset of 1,163,401 ECGs taken from 189,539 patients.
Using a deep learning and a discrete-time survival model, researchers were able to create a patient-specific survival curve with a single ECG, allowing the AIRE platform to predict not only risk of mortality but also time-to-mortality.
The platform was validated in five diverse, transnational cohorts from the USA, Brazil, and the UK, including volunteers, primary care patients, and secondary care patients.
Researchers found the model was able to identify the risk of death in the ten years following the ECG (from high to low) in 78% of cases, and was also able to predict future ventricular arrhythmia, future atherosclerotic cardiovascular disease, and future heart failure.
‘Through phenome-wide and genome-wide association studies, we also identified candidate biological pathways for the prediction of increased risk, including changes in cardiac structure and function, and genes associated with cardiac structure, biological ageing, and metabolic syndrome,’ they wrote.
They concluded that clinicians could act on AIRE’s predictions to provide targeted, personalised and earlier intervention.
‘AIRE is an actionable, explainable, and biologically plausible AI-ECG risk estimation platform that has the potential for use worldwide across a wide range of clinical contexts for short-term and long-term risk estimation,’ they said.
Lead author Dr Arunashis Sau, an academic clinical lecturer at Imperial College London’s National Heart and Lung Institute, and cardiology registrar at Imperial College Healthcare NHS Trust, said compared with cardiologists the AI model could detect more subtle detail in the ECGs.
‘It can ‘spot’ problems in ECGs that would appear normal to us, and potentially long before the disease develops fully,’ he said.
‘Our analysis shows that the AI can tell us a lot about not only about the heart but also what is going on elsewhere in the body and may be able to detect accelerated ageing.’
Dr Sau acknowledged it was necessary to see how the model performed in the healthcare system, but suggested it was possible that in the future, the technology could be used in a wearable device that provided doctors with continuous remote monitoring and a potential alert system.
Senior study author Dr Fu Siong Ng, Reader in Cardiac Electrophysiology at the National Heart & Lung Institute at Imperial College London, said the work had shown the AI model was a credible and reliable tool that could, in future, be programmed for use in different areas of the NHS to provide doctors with relevant risk information.
‘This could have a positive impact on how patients are treated, and ultimately improve patient longevity and quality of life, he said.
Dr Ng, who is also a consultant cardiologist at Imperial College Healthcare NHS Trust and Chelsea and Westminster Hospital NHS Foundation Trust, said the technology could also reduce waiting lists and allow more efficient allocation of resources.
Trials of the model are planned to start by mid-2025 in hospitals across Imperial College Healthcare NHS Trust and Chelsea and Westminster Hospital NHS Foundation Trust.
Participants are set to be recruited from outpatient clinics and from inpatient medical wards.
30th May 2024
Research from King’s College London (KCL) has found that exposing children to peanuts from infancy to age five reduced the rate of peanut allergy in adolescence by 71%.
The new study adds to evidence that the early introduction of peanuts and peanut products in infancy induces long-term tolerance and protects adolescents from allergy.
The researchers say that early peanut consumption will result in peanut allergies plummeting and could prevent more than 100,000 new cases of peanut allergy every year worldwide.
The study, which was sponsored and co-funded by the US National Institutes of Health’s National Institute of Allergy and Infectious Diseases (NIAID), is published in the journal NEJM Evidence.
In western countries such as North America, the UK, western Europe and Australia, rates of peanut allergy amongst young people are currently around 2% but are rising. Life-threatening allergic reactions and conflicting health advice have led parents and caregivers to be fearful of introducing peanuts into young children’s diets.
The trial, known as the LEAP-Trio Study, built on the findings of the Learning Early About Peanut Allergy (LEAP) clinical trial. The initial study looked at the impact of introducing peanuts to children from infancy to five years of age, using a trial group, who were exposed to peanuts and a control group who were not.
Findings showed that children who ate peanuts and peanut products from an early age had an 81% reduced risk of allergy at age five.
Building on this work, the researchers followed both groups of children between the ages of six and 12. Children could eat peanuts in whatever amount and frequency they wanted during that period. The adolescents were tested for peanut allergy primarily through an oral food challenge, which involved giving participants gradually increasing amounts of peanut in a carefully controlled setting to determine if they could safely consume at least 5g of peanut, the equivalent of more than 20 peanuts.
The study team also surveyed participants about their recent patterns of peanut consumption, which were verified through measurements of peanut in dust from participants’ beds, a technique previously validated by LEAP investigators.
The early avoidance group showed a peanut allergy level of 15.4% by the age of 12. This was compared to 4.4% in the children who had eaten peanuts from an early age. Following the children through to age 12 showed that regular, early peanut consumption reduces the risk of peanut allergy in adolescence by 71% compared to early peanut avoidance.
Lead investigator Professor Gideon Lack, head of the children’s allergy service at Guy’s and St Thomas’ NHS Foundation Trust and professor of paediatric allergy at KCL, said: ‘Decades of advice to avoid peanuts has made parents fearful of introducing peanuts at an early age. The evidence is clear that early introduction of peanut in infancy induces long-term tolerance and protects children from allergy well into adolescence. This simple intervention will make a remarkable difference to future generations and see peanut allergies plummet.’
Throughout childhood, the overall consumption of peanuts and peanut products was higher in the group who had been exposed to peanuts between infancy and age five. However, during the period of six to 12 years of age, peanut consumption varied widely between the two groups and included periods of not eating peanuts for some participants in both groups. The findings suggest that the protective effect of early peanut consumption lasts, without the need to eat peanut products consistently throughout childhood and early adolescence.
Professor George Du Toit, co-lead investigator and professor in paediatric allergy at Guy’s and St Thomas’ hospitals and KCL, said: ‘This is a safe and highly effective intervention which can be implemented as early as four months of age. The infant needs to be developmentally ready to start weaning, and peanut should be introduced as a soft pureed paste or as peanut puffs.’
A version of this article was first published in our sister publication Nursing in Practice.
29th May 2024
Patients with stable coronary artery disease (CAD) but without standard modifiable cardiovascular risk factors (SMuRFs) have a substantial but significantly lower risk of long-term cardiovascular events than people with risk factors, an observational study has found.
Previous research found patients without SMuRFs presenting with first myocardial infarction (MI) had a higher in-hospital mortality than patients with risk factors, researchers wrote in the European Heart Journal.
They said the term ‘SMuRF-less’ was recently coined to stress the need for further research on cardiovascular outcomes in this group who did not have the standard risk factors of diabetes, dyslipidaemia, hypertension, and smoking.
To explore the long-term outcomes of SMuRF-less patients with stable CAD, the researchers analysed data from CLARIFY, an observational study of 32,703 patients with stable CAD enrolled between 2009 and 2010 in 45 countries in Europe, Asia, America, the Middle East, Australia, and South Africa.
Among 22,132 patients with complete risk factor and outcome information, 977 (4.4%) were classed as SMuRF-less.
Age, sex, and time since CAD diagnosis were similar across groups, the researchers reported.
SMuRF-less patients had a lower five-year rate of cardiovascular death or non-fatal MI than patients with risk factors (5.43% vs 7.68%), researchers reported.
All-cause mortality and major adverse cardiovascular events (cardiovascular death, non-fatal MI, or non-fatal stroke) were also lower in SMuRF-less patients, they found.
Senior author Professor Phillipe Gabriel Steg, professor of cardiology at the University of Paris, France, and colleagues said the risk of adverse cardiovascular outcomes increased steadily with the number of risk factors.
‘Standard modifiable cardiovascular risk factor-less status appeared more protective in women than in men,’ Professor Steg and co-authors noted.
However, they stressed the study did not question the need for SMuRF-less patients with stable CAD to receive guideline-based therapy, adding that SMuRF-less patients remained at a substantial risk of cardiovascular events.
‘While the risk was lower in SMuRF-less patients, they still experience a substantial risk of cardiovascular events; thus efforts should be made to achieve higher rates of implementation of evidence-based therapies,’ Professor Steg and colleagues wrote.
The study found SMuRF-less patients were less likely to be prescribed statins, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and beta-blockers.
‘It remains crucial to improve the visibility of the SMuRF-less population in future clinical trials and guidelines,’ Professor Steg and colleagues concluded.
The previous body of research of SMuRF-less patients had focused on in-hospital mortality after first-presentation MI, with the paradoxical finding that SMuRF-less patients might experience more adverse outcomes after first-presentation MI.
However, the causes of the poor prognosis of SMuRF-less patients in the immediate post-infarction period as reported in previous research were not explored in the present study, the study authors noted.
‘SMuRF-less patients may have specific causes of MI (dissection, embolism, spasms, and the use of toxic substances), which can be difficult to manage during the acute phase but are associated with fewer comorbidities,’ the researchers suggested.
‘Future research efforts should focus on the early post-MI period, when the vulnerability of SMuRF-less patients seems to be at stake.’
The study was funded by pharmaceutical firm Servier, but the authors said the sponsor had no role in the study design or management.
22nd February 2024
A study shows that gout not only increases the risk of a broad range of cardiovascular diseases but also leads to a higher prevalence of other health conditions, including chronic kidney disease, high blood pressure and an increased body mass index (BMI).
Researchers from the Universities of Oxford, Glasgow, and KU Leuven in Belgium found that having gout is linked with a 58% higher risk of cardiovascular disease. This risk increased further for females and people under the age of 45.
Gout has previously been linked with an increased risk of cardiovascular disease, but this study, published in The Lancet Rheumatology, is the first to link gout with a broader range of health issues.
The condition can be extremely painful and is caused by a build-up of uric acid in the body, which crystallises around joints, causing pain, swelling and redness. Gout is one of the most common types of inflammatory arthritis in the world and is particularly common in older men.
Using the Clinical Practice Datalink, the researchers were able to analyse the electronic health records of over 860,000 people from the UK and Europe. Over 152,000 individuals who had gout were identified, alongside more than 700,000 matched population controls.
People with gout were found to be at a higher risk of cardiovascular disease than those without the condition. In particular, women with gout had an 88% higher relative risk of heart disease. For men, the risk was 49% higher for those with gout when compared to the control group.
The increased risk was seen across all of the twelve heart conditions analysed in the study, including heart failure, ischaemic heart disease, arrhythmias, valve diseases, and venous thromboembolism and was amplified in younger patients.
People under 45 years of age who had gout were found to have more than twice the risk of cardiovascular disease than a similar-aged person without gout.
BMIs were also found to be higher in patients with gout, and higher rates of other health conditions, such as chronic kidney disease and high blood pressure, were also observed.
Dr Nathalie Conrad, a senior author on the paper from KU Leuven and an honorary research fellow at the University of Oxford and the University of Glasgow, said: ‘The present results complement a now large body of evidence of substantial cardiovascular risks associated with gout, as well as other immune-mediated inflammatory conditions.
‘To date, these conditions are less commonly considered in cardiovascular disease prevention guidelines and risk scores, nor are there specific prevention measures for these patients.
‘These data suggest this might need to change, and the clinical community may need to consider cardiovascular disease screening and prevention as an integral part of the management of gout.’
The researchers highlight the importance of screening for and managing a range of cardiovascular diseases in people with gout.
Dr Lyn Ferguson, from the University of Glasgow, added: ‘Gout could be considered a metabolic condition, and management should include addressing the heart and body weight alongside joints.’
A version of this article was originally published by our sister publication Nursing in Practice.
30th August 2023
Recreational drug (RD) use was detected via the urine samples of approximately one in 10 patients (11%) admitted to an intensive cardiac care unit, according to the findings of a multi-centre French study.
Published online in the journal Heart, the study also found the presence of such drugs was independently associated with a higher risk of in-hospital major adverse events (MAEs).
The use of RDs is already known to be associated with a higher risk of cardiac arrhythmias. However, there is some uncertainty over the prevalence of RD use among patients admitted to hospital with heart problems, or to what extent this affects the likely course of their condition.
The researchers aimed to assess the prevalence of RD use and its association with in-hospital MAEs in patients admitted to intensive cardiac care units. Among those admitted, systematic screening for RDs was performed by prospective urinary testing.
The primary outcome was the prevalence of RD detection. In-hospital MAEs were defined as death, resuscitated cardiac arrest or haemodynamic shock.
In 499 consecutive patients with a mean age of 63 years (70% male), 161 (11%) had a positive test for a RD, including cannabis (9.1%), opioids (2.1%), cocaine (1.7%), amphetamines (0.7%) and MDMA (0.6%). Despite these findings, only 57% of those testing positive declared recreational drug use.
Patients who used RDs exhibited a higher MAE rate than non-users (13% vs 3%, respectively, p < 0.001).
After adjustment for comorbidities, RD use was independently associated with a higher rate of in-hospital MAEs (odds ratio, OR = 8.84, 95% CI 4.68 – 16.7, p < 0.001). In fact, cannabis, cocaine and MDMA, when assessed separately, were independently associated with in-hospital MAEs.
Multiple drug detection was frequent (28% of positive patients) and also associated with an even higher incidence of MAEs (OR = 12.7, 95% CI 4.80 – 35.6, p < 0.001).
4th July 2023
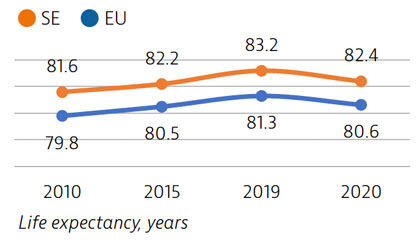
Life expectancy in Sweden is among the highest in the EU, although it declined by nearly one year in 2020 as a result of the Covid-19 pandemic. The healthcare system generally performs well in providing good access to high-quality care.
However, challenges persist in providing equal access to care for the population living in different regions, ensuring timely access to care, achieving greater care coordination for people with chronic diseases and improving the quality of long-term care.
Life expectancy at birth was 82.4 years in 2020 – almost two years above the EU average – but it fell by almost one year in 2020 because of the high number of deaths from Covid-19. More than two thirds of Covid-19 deaths were among people aged 80 and over.
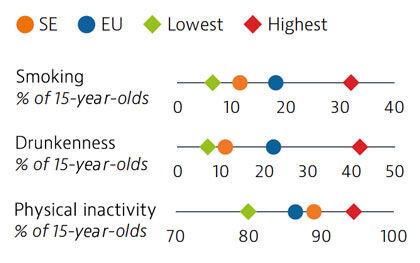
Smoking rates among adults in Sweden are among the lowest in EU countries, but use of other tobacco products such as snuff is common. Overall alcohol consumption per adult has decreased over the past decade and is much lower than the EU average. Adolescents in Sweden also report low rates of smoking and excessive alcohol intake, but high rates of physical inactivity.
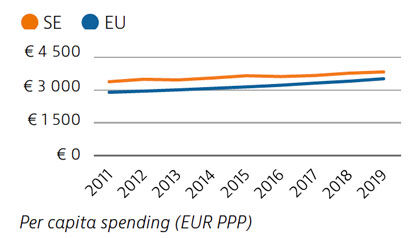
Health spending per capita in Sweden was the fourth highest in the EU in 2019, and the third highest in terms of health spending as a share of GDP. Most health spending is publicly funded (85%). The growth rate in health spending was relatively modest in the years prior to the pandemic, but the government increased spending on health in 2020 and 2021 in response to Covid-19.
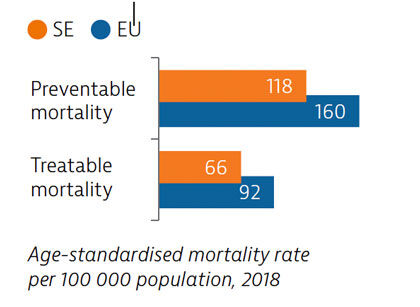
Sweden had low rates of mortality from preventable and treatable causes in 2018, pointing to a generally effective public health and healthcare system under normal circumstances.
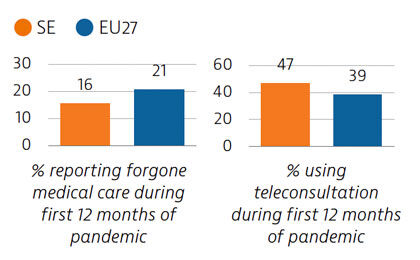
During the first year following the pandemic, one in six people in Sweden reported some unmet needs for medical care, which is slower than the EU average. The use of teleconsultations increased quickly in Sweden during the pandemic to maintain access to care.
Sweden tried to balance protection of people’s health and protection of economic and social activities in managing the Covid-19 crisis. While fewer restrictions were imposed, particularly during the first wave, the death toll was high compared with other Nordic countries. By end of August 2021, 58% of the population had received two doses or the equivalent – slightly more than the EU average.
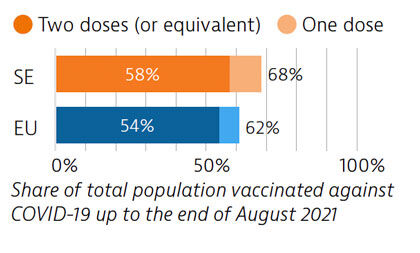
OECD/European Observatory on Health Systems and Policies (2021), Sweden: Country Health Profile 2021, State of Health in the EU, OECD Publishing, Paris/European Observatory on Health Systems and Policies, Brussels.