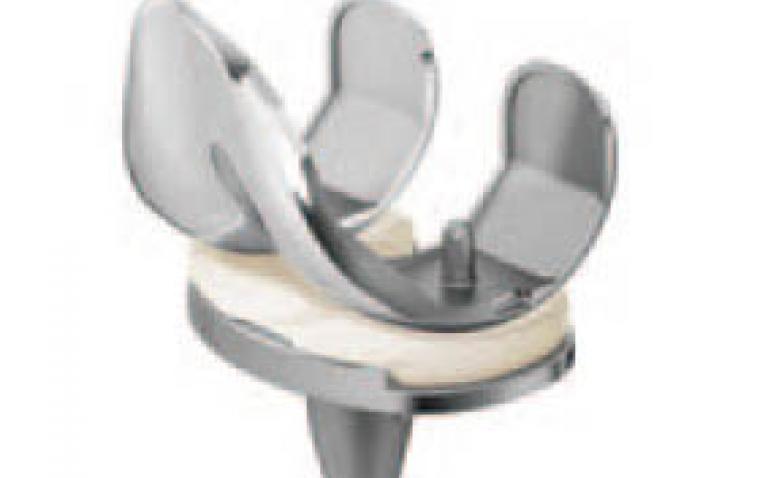
DePuy Synthes Joint Reconstruction*, a global leader in joint replacement, today announced the introduction of two new technologies for the ATTUNE® Knee System—the ATTUNE Rotating Platform and the Medialized Anatomic Patella.
The announcement was made in conjunction with the 15th European Federation of National Associations of Orthopaedics and Traumatology (EFORT) congress, a combined programme in partnership with the British Orthopaedic Association, held in London, UK.
“The new ATTUNE Rotating Platform Knee and the Medialized Anatomic Patella provide additional options for patient care,” said Gary Lancaster, Knee Marketing Director, DePuy Synthes. “Offering the ATTUNE Knee with both fixed bearing and rotating platform options is important from the standpoint of surgeon preference and expertise, as well as individual needs of patients.”
The advantage of rotating platform knees is that the bearing can rotate as the knee flexes, which allows for a more natural motion and may also reduce the stress and wear on the implant. The ATTUNE Rotating Platform Knee builds on DePuy Synthes Joint Reconstruction’s strong leadership in rotating platform knees and combines that expertise with the proven technologies of the ATTUNE Knee, which is designed to enhance stability and motion.
Studies show that between 10–20%t of knee replacement patients are not completely satisfied with their knee replacement. A major contributing factor to this is anterior knee pain in the area of the patella. The Medialized Anatomic Patella was created to help address this need as it is designed to wrap around the knee in a more natural way and improve patellar tracking.
The ATTUNE Knee System, the largest research and development project in the history of DePuy Synthes Joint Reconstruction, was designed to provide better range of motion and address the unstable feeling some patients experience during everyday activities, such as stair descent and bending. The launch of the ATTUNE Rotating Platform Knee follows last year’s launch of the ATTUNE Fixed Bearing Knee. To date, more than 41,000 ATTUNE Knee implants have been provided for patients worldwide.
ATTUNE Rotating Platform Knee
The Rotating Platform design increases the level of conformity to provide stability while delivering freedom of mobility. In addition, the Rotating Platform design gives the tibial insert the freedom to self-align and track with the femoral component throughout the range of motion, allowing surgeons the ability to position the rotating platform tibial base on the proximal tibia for maximum bony coverage. The ATTUNE Rotating Platform Knee builds on DePuy Synthes Joint Reconstruction’s heritage, including the LCS® Knee System and the SIGMA® Rotating Platform Knee System. More than one million Rotating Platform knees have been provided for surgeons and patients around the world.
ATTUNE Medialized Anatomic Patella
The Medialized Anatomic Patella works with the ATTUNE Knee femoral components. The Medialized Anatomic Patella is unique to DePuy Synthes Joint Reconstruction and is compatible with both ATTUNE Fixed Bearing and Rotating Platform Knees. The Medialized Anatomic Patella is designed to have more natural sagittal plane kinematics than traditional dome style patella components. These more natural kinematics are designed to reduce soft tissue interaction with the femoral component and thereby help prevent soft tissue irritation. Also, the unique kinematics of the Medialized Anatomic Patella are designed to increase quadriceps efficiency in deep flexion, allowing the knee to more easily flex and extend.
About DePuy Synthes Joint Reconstruction
DePuy Synthes Joint Reconstruction, a global leader in hip, knee and shoulder replacement, is part of DePuy Synthes Companies of Johnson & Johnson, the largest provider of orthopaedic and neurological solutions in the world. For more information, visit www.depuysynthes.com.
*DePuy Synthes is a trading division of DePuy International Ltd.
The third party trademarks used herein are the trademarks of their respective owners.
References
- McNulty DE, Swope SW, Auger DD, Smith T. The effect of crosslinking UHMWPE on in vitro wear rates of fixed and mobile bearing knees. ASTM STP 1445. Gsell, R. et al. American Society for Testing and Materials, West Conshohocken, PA. Available online at www.astm.org (2004).
- Baker PN, van der Meulen JH, Lewsey J, Gregg PJ. The role of pain and function in determining patient
- Sensi L, Buzzi R, Giron F, De Luca L, Aglietti P. Patellofemoral function after total knee arthroplasty: gender related differences. J Arthroplasty. 2011; 26(8): 1475–80.
- Data on file at DePuy Orthopaedics, Inc., 2014
- Data on file at DePuy Orthopaedics, Inc., 2007.