Globalisation affects all areas of life, including healthcare. For globalisation to have a positive influence, it is necessary to have global standards and units to which all measurements are traceable. We are familiar with global standards for time, temperature, mass and length and these standards, coupled with Système Internationale (SI) units, ensure that these parameters can be measured anywhere in the world with a small measurement uncertainty, enabling the safe transfer of data between global stakeholders.
The same principle should apply to measurements made, and results used, in healthcare.
Laboratory medicine is an essential clinical specialty providing users with pivotal information for the prevention, diagnosis, treatment and management of health and disease. Laboratory medicine has several specialisms including clinical chemistry, genetics, haematology, immunology, microbiology and transplantation. Laboratory medicine results provide information that impacts a high percentage of clinical decisions in healthcare. This central role means that laboratory medicine specialists have a professional responsibility to provide a high-quality service that is optimised to the needs of the patient.1
A high percentage of laboratory medicine results are obtained using commercially produced in vitro diagnostic (IVD) test systems. The global market for these IVD products was estimated at $61 billion in 2016, with a growth rate of 4.6% per annum.2 Many companies are involved in producing IVD test systems. Unfortunately, it is common for the results obtained from different IVD test systems for the same analyte to be unacceptably high. Therefore, one increasingly important quality objective is to ensure that patient test results are traceable (equivalent) between different methods, laboratories and healthcare systems over time.3
Equivalence of test results is being achieved through the process of harmonisation in laboratory medicine. The aim of harmonisation is to provide accurate, actionable and transferable patient results, which can facilitate improved clinical outcomes and patient safety. Harmonisation in laboratory medicine has a wide scope. It can be applied across the total testing process of laboratory medicine, including requests, samples, measurements and reports.4 The many dimensions of harmonisation require active involvement at local, national and international levels.5
The importance of reducing between-method variability
There are several reasons why efforts should be made to reduce between-method variability in laboratory medicine.4 These include:
- Patient safety: Differences in practice and variability of results put patients at risk. Harmonisation of patient results should contribute to improved clinical outcomes
- Patient empowerment: Healthcare is increasingly patient-centred. Patients expect results from laboratories and from self-testing to be identical and method independent
- Public confidence: The public will be reassured by the knowledge that patient results are accurate and transferable between laboratories
- Laboratory accreditation: The ISO 15189:2012 standard used for medical laboratory accreditation requires trueness of measurement and metrological traceability
- Clinical guidelines: The successful implementation of clinical practice guidelines often links patient management to specific values or changes in patient results
- Clinical governance: Differences between patient results leads to concerns about the quality and professionalism of the service that is provided
- Consolidation and networking: Laboratory networks providing services to both primary and secondary care should be able to provide similar results from any laboratory site
- Informatics: Laboratory information systems and hospital information systems will only be able to share and transfer results if they are harmonised
- Electronic patient record: National electronic patient records require that patient results may be inserted from any laboratory and so they should be transferable.
Reasons why methods might give different results on a patient sample
Patients and the public naturally assume that all methods for measurement of a single analyte will give the same result on a patient sample. For some simple analytes, such as plasma glucose, the results will be very similar. However, for more complex analytes, the results might vary considerably. There are many potential reasons for these differences, and these can be summarised as follows:
- Companies: There are many IVD method manufacturers around the world. Their individual methods may have different specimen requirements; employ different method designs and use different signal detection systems. Variability may also be introduced by local modification to a company product
- Components: Methods may use different calibrators; different enzymes and substrates; different antigens and antibodies; and a variety of other reagents
- Conditions: Different methods have variability in reaction time; temperature, pH and often use different software and curve fits to derive results
- Common target: Although methods will quote figures for imprecision and accuracy (trueness) these are of limited value unless they can be related to a common, international reference system.
Traceability in laboratory medicine
Metrology is the science of measurement. The application of metrology provides the key to reducing measurement variability by facilitating the adoption of international reference systems to enable alignment from different methods. The increasing application of metrological traceability in laboratory medicine (TLM) to underpin those reference systems is reducing the variables that are responsible for methods giving different results.6 TLM is an important area of laboratory science that is often poorly understood. Achieving TLM is a global multi-stakeholder, cooperative activity involving metrologists; international standards organisations; scientific and clinical experts from international professional bodies; healthcare regulators; and the IVD industry.7
Metrological traceability is defined as the property of a measurement result, which can be related to a reference standard through a documented unbroken chain of calibrations.
The principles of a reference measurement system for establishing metrological traceability are described in the relevant International Standards Organisation (ISO) document.8 The components of a reference measurement system comprise reference materials (calibrators) and reference measurement procedures (methods), both of which exist at different hierarchical levels.
The inter-relationship between the components of a reference measurement system may be described in the metrological traceability chain.8
Figure 1 depicts this traceability chain with higher order reference materials and measurement procedures at the top and lower order towards the bottom. At the top of the chain is the definition of the measurand, the substance intended to be measured, expressed in SI units. The hierarchy of reference materials and measurement procedures is depicted by the rising metrological traceability arrow. Descent through the traceability chain is accompanied by increasing measurement uncertainty as depicted by the downward arrow. Figure 1 also depicts the contributions to TLM of the metrology institute or reference laboratory; the IVD method manufacturer; and the routine service laboratory.
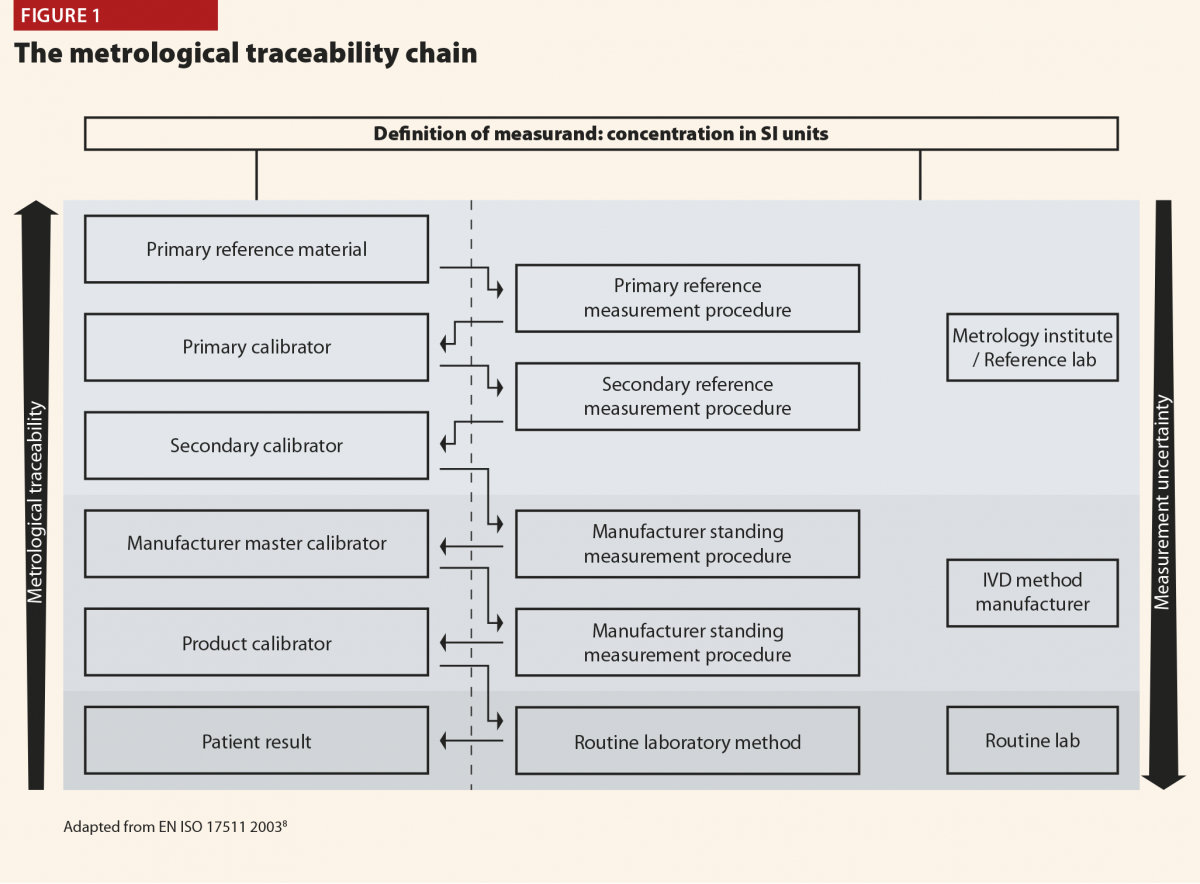
The relevance of TLM may be explained by considering the measurement of plasma glucose, one of the most common biomarker tests. There are many IVD methods for plasma glucose, but they all comply with the traceability chain so that the result obtained in the routine laboratory can be traced back to a primary reference material of pure glucose. As a result, there is excellent agreement between methods for plasma glucose, and results from elsewhere can be interpreted with confidence.
For structurally simple molecules, like many of those measured routinely in clinical chemistry, pure substance is available, and it is possible to have a complete unbroken traceability chain. Even for some protein molecules it is possible to achieve full metrological traceability by using a unique, signature peptide as the primary reference material. Consequently, there are a growing number of important biomarkers where method standardisation has been achieved in a way comparable to plasma glucose.
Regrettably, most of the biomarkers measured in laboratory medicine are not structurally simple molecules. For example, complex proteins, nucleic acids, viruses and bacteria are clinically important biomarkers that may not be available as pure substance and reference methods of measurement are unlikely to be available. In these circumstances full metrological traceability is not possible. The adoption of international conventional calibrators, with values assigned by experts, represents traceability part of the way up the chain and the use of such calibrators provides a reference against which methods can be harmonised to reduce between method variability.
The Joint Committee for Traceability in Laboratory Medicine (JCTLM)
The JCTLM was established in 2002 through a declaration between the International Bureau of Weights and Measures (BIPM), the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) and the International Laboratory Accreditation Cooperation (ILAC) in response to the implementation of the European Community Directive 98.79/EC on in vitro medical devices. JCTLM currently has >50 members from 20 countries and its membership is growing rapidly.
The aim of the JCTLM is to support world-wide comparability, reliability and equivalence of measurement results in laboratory medicine, for improving health care and facilitating national and international trade in IVD devices, by:
- Promoting the concept of traceability of measurement results
- Evaluating reference materials, reference measurement procedures and reference measurement services for laboratory medicine with respect to conformity with appropriate international standards;
- Facilitating the identification and prioritisation of measurands requiring international traceability and comparability and thereby encouraging appropriate organisations to accept responsibility for the development of suitable reference methods and measurement procedures and certified reference materials;
- Producing educational materials and activities promoting the value of traceability in laboratory medicine and raising awareness amongst stakeholders;
- Encouraging the IVD industry to apply the agreed reference measurement systems.
The main output of the JCTLM is the global database of higher order reference materials; reference measurement methods/procedures; and reference measurement services.9 This database is freely available to any user. In July 2019, the JCTLM database contained entries for some 300 reference materials and almost 200 reference methods. The supporting output of the JCTLM is a website that contains freely available educational support materials, including webinars, publications and presentations from meetings.10
Challenges in implementing traceability in laboratory medicine at a global level
As the content of the JCTLM database demonstrates, significant progress has been made in the application of TLM to produce reference materials and measurement procedures. However, more than 1000 analytes are routinely measured across laboratory medicine, and there are significant challenges in implementing global TLM for many of these analytes. These challenges include:
- Geographical, cultural and language differences
- The variable use of SI units
- The growing biomarker repertoire, including many complex analytes
- A lack of global coordination and leadership.
These challenges are being addressed but progress is largely dependent on voluntary effort and so it is too slow to meet the needs of patients. A ‘call to arms’ has been issued to establish a global forum to manage TLM.11
A role for hospital and laboratory managers in implementing traceability in laboratory medicine
While much of the effort involved in achieving TLM is a global endeavour, there are steps that can be taken by hospital and laboratory managers to protect patients and to educate the laboratory users for whom they are responsible. These steps include:
- Determine the traceability status of the methods currently used and understand the measurement uncertainty involved. This information will be available in the small print of the instructions for use provided by the IVD manufacturer
- Select new or replacement methods where the traceability status is established
- Alert users to analytes where there is known between method variability with a warning that data from other laboratories may not be transferable
- Ensure that all laboratory methods participate in reputable external quality assessment (EQA) schemes and evaluate performance on a regular basis
- Refer staff and users to the TLM educational support available from www.jctlm.org.
References
1 Beastall GH. Adding value to laboratory medicine: a professional responsibility. Clin Chem Lab Med 2013;51:221–8.
2 IVD market overview. Allied Market Research 2018. www.alliedmarketresearch.com/ivd-in-vitro-diagnostics-market (accessed August 2019).
3 Greenberg N. Update on current concepts and meanings in laboratory medicine – standardization, traceability and harmonization. Clin Chim Acta 2014;432:49–54.
4 Plebani M. Harmonization in laboratory medicine: the complete picture. Clin Chem Lab Med 2013;51:741–51.
5 Plebani M. Harmonization in laboratory medicine: requests, samples, measurement and reports. Crit Rev Clin Lab Sci 2016; 53:184–96.
6 White GH. Metrological traceability in clinical biochemistry. Ann Clin Biochem 2011; 48: 393-409
7 Beastall GH, Brouwer N, Quiroga S, Myers GL. Traceability in laboratory medicine: a global driver for accurate results for patient care. Clin Chem Lab Med 2017;55:1100–08.
8 ISO 17511: 2003 In vitro diagnostic medical devices – measurement of quantities in biological samples – metrological traceability of values assigned to calibrators and control materials ISO, Geneva, Switzerland; 2003.
9 JCTLM. JCTLM database of reference materials and measurement procedures. www.bipm.org/jctlm/ (accessed August 2019).
10 JCTLM. Working Group on traceability, education and promotion.www.bipm.org/cc/JCTLM/Allowed/2015-11-30/3_G-Beastall_JCTLM-WG-TEP_2015.pdf (accessed August 2019).
11 Cobbaert C, Smit N, Gillery P. Metrological traceability and harmonization of medical tests: a quantum leap forward is needed to keep pace with globalization and stringent IVD-regulations in the 21st century. Clin Chem Lab Med 2018;56:1598–602.










