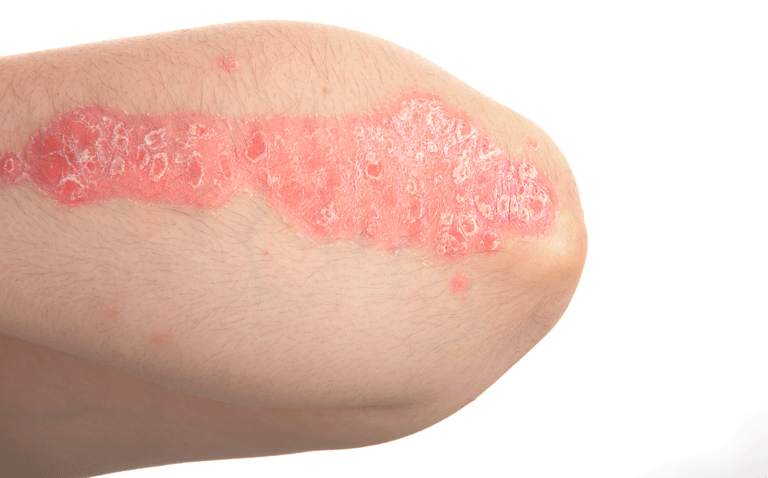
Tapinarof cream was found to be effective in two Phase III, randomised trials for patients with moderately severe plaque psoriasis
Topical therapy with tapinarof 1% cream has been found to be effective for plaque psoriasis over a 12 week period in patients with moderately severe disease. This was the conclusion of a study by a team from the Icahn School of Medicine at Mount Sinai, New York, US.
Psoriasis is a common, chronic, recurrent, immune-mediated disease of the skin and joints that affects around 125 million people worldwide equating to between 2 and 3% of the population. Although patients with severe psoriasis can be managed with biologics, the majority of patients have mild-to-moderate psoriasis that can be adequately controlled with topical therapy; however, adherence to topical therapy is often suboptimal.
Tapinarof is a novel, non-steroidal, topical aryl hydrocarbon receptor-modulating agent and its mode of action is attributed to specific binding and activation of AhR, a ligand-dependent transcription factor, leading to the down-regulation of pro-inflammatory cytokines, including interleukin 17, and regulation of skin barrier protein expression to promote skin barrier normalisation.
In the present analysis, the authors provide results obtained from two identical, randomised, double-blind, Phase III trials, PSOARING 1 and PSOARING 2, which assessed tapinarof 1% cream in adult patients (aged 18 years and over) with mild to moderate plaque psoriasis. Patients were randomised 2:1 (treatment: placebo) and assessed using the Physician’s Global Assessment (PGA) score which ranged from 0 (clear) to 4 (severe) and treatments were applied once daily. The primary efficacy endpoint was a PGA response, defined as a PGA score of 0 (clear) or 1 (almost clear) and a decrease from baseline of at least 2 points on the PGA scale. The main secondary outcome was the proportion of patients achieving a PASI 75 (which represents a 75% reduction in disease severity) and both assessments were made at week 12. The main patient reported outcome was a change of at least 4 points in the Peak Pruritus Numeric Rating Scale (PP-NRS) based on an 11-point (where 0 is no itch and 11 is the worst imaginable itch).
Findings
A total of 510 and 515 patients were enrolled in both trials with a mean age of approximately 49 years and the proportion of male patients ranging from 50.6 to 62.6%. At entry, between 79.7 and 84% of participants had a PGA score of 3 (i.e., moderate disease severity) and a PP-NRS score of approximately 5.7.
The primary end point was achieved by 35.4% and 40.2% of patients in the tapinarof groups in the two trials compared to 6% and 6.3% in those assigned to placebo (p < 0.001 for both trials). In addition, 36.1% and 47.6% of the intervention groups achieved a PASI 75 compared to 10.2% and 6.9% in the placebo groups (p < 0.001 for both trials). However, while reductions in PP-NRS scores were numerically higher in both tapinarof groups this difference was not reported as statistically significant.
The authors reported that overall, adverse events in the tapinarof group were higher 50.3% vs 22.4% in PSOARING 1 and a similar proportion was seen in the second trial. The most frequent adverse events were folliculitis, nasopharyngitis, contact dermatitis and headache, all of which were more common with tapinarof.
The authors concluded that while tapinarof was more effective than placebo, larger and longer trials were required to evaluate its efficacy compared with existing topical psoriasis treatments.
Citation
Lebwohl MG et al. Phase 3 Trials of Tapinarof Cream for Plaque Psoriasis N Engl J Med 2021