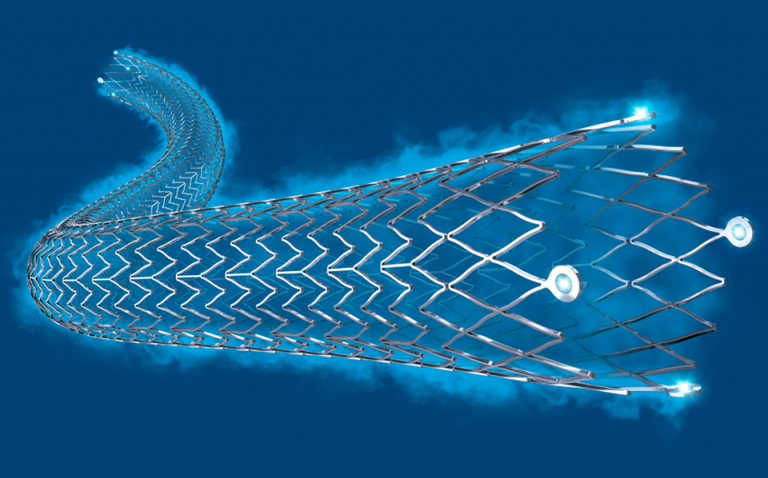
Boston Scientific announced that the Eluvia™ Drug-Eluting Vascular Stent System has received CE Mark and is commencing commercialisation immediately in the European Union and other countries where CE Mark is recognised.
The Eluvia Stent System is designed to restore blood flow in the peripheral arteries above the knee – specifically the superficial femoral artery and proximal popliteal artery. The stent features a unique drug–polymer combination intended to facilitate sustained release of the drug (paclitaxel) that can prevent narrowing (restenosis) of the vessel, often the cause of pain and disability for patients diagnosed with peripheral artery disease.
CE Mark approval was based on data from the MAJESTIC trial, a prospective, multicentre clinical trial that assessed the safety and performance of the Eluvia Stent System and reflected a primary patency rate of more than 96%.1 The MAJESTIC trial results represented the highest 12-month primary patency reported for an interventional treatment of femoropopliteal artery lesions among comparable trials.
“The exceptional 12-month results presented in the MAJESTIC trial, which included a high percentage of patients with complex lesions, demonstrate that this technology is a safe and efficacious solution for patients needing stents for the treatment of peripheral artery disease,” said Professor Stefan Müller-Hülsbeck, MD, PhD, principal investigator at the Vascular Center Diako Flensburg and head of the Department of Diagnostic and Interventional Radiology/Neuroradiology, Academic Hospitals Flensburg, Germany. “The approval is a testament to the strength of the data, and will be welcome news to physicians and patients who have not previously had access to a polymer based, drug-eluting stent, specifically developed for the superficial femoral and proximal popliteal arteries.”
Boston Scientific received an Investigational Device Exemption (IDE) to conduct a global, prospective trial called the IMPERIAL trial, which will assess the safety and efficacy of the Eluvia Stent System compared to the Zilver® PTX® Stent manufactured by Cook Medical. Enrolment began in Q4 last year and the study will include approximately 485 patients in 75 sites worldwide.
“The availability of the Eluvia Stent System to European patients, paired with the expansion of our existing clinical program, demonstrates the momentum of our drug-eluting portfolio in combatting peripheral artery disease,” said Jeff Mirviss, senior vice president and president, Peripheral Interventions, Boston Scientific. “Our legacy with drug-eluting technology combined with our commitment to further advance treatment options for peripheral artery disease, enables Boston Scientific to continue bringing ground breaking solutions for patients around the world.”
Reference:
- Primary patency defined as duplex ultrasound peak systolic velocity ratio < 2.5 and absence of TLR or bypass; data reflect actual values (not Kaplan Meier estimates).