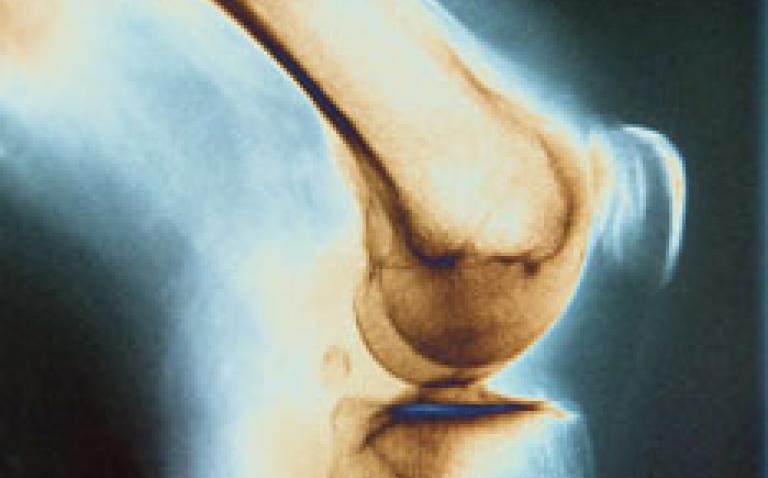
An arthritis charity yesterday accused the UK government’s health watchdog of “penny-pinching” in its decision not to make an anti-TNF drug available on the NHS.
“Anti-TNFs can slow down the progress of rheumatoid arthritis. We hear a lot about patient ‘choice’ but where is it in the decision to limit treatment options in this way? But any supposed saving is false – the human cost alone is enormous, not to mention the cost to society and the economy,” said Arthritis Care chief executive Neil Betteridge.
“The decision means that people now only have one option (rituximab) if their anti-TNF doesn’t work – and if rituximab fails them too, their disease will progress, causing irreversible damage to their joints, disability, poverty, and a significantly shortened life expectancy.
“It just robs Peter to pay Paul as they will need higher levels of NHS intervention, more in-patient bed days, more orthopaedic surgery, and probably end up leaving their jobs and having to claim incapacity benefit instead of being active, productive members of UK Inc,” he said.
The National Institute for Health and Clinical Excellence (NICE) delivered its decision yesterday on the sequential use of anti-TNFs, which, if allowed, would have meant that people in England and Wales could try another drug in the same class if their initial treatment did not work.
As each person reacts differently, when prescribing, doctors cannot predict which drug will best suit an individual. But it is known that four out of five people respond to a second anti-TNF therapy, according to Arthritis Care.