Viroclinics takes the opportunity to contribute a virological Continuing Education topic for the November 2013 issue of Medical Laboratory Observer (MLO) as a contribution to the ongoing improvements of influenza diagnostic tests, vaccines and antiviral drugs.
Antiviral drug against influenza are still urgently needed
Anti-influenza antiviral drugs are not a substitute for vaccines. They are used in addition to vaccines in public health planning for the control of influenza. The antiviral drugs have been approved for treatment of acute uncomplicated influenza and are used as part of pandemic preparations by government.
Virus shedding and reduce of transmissibility
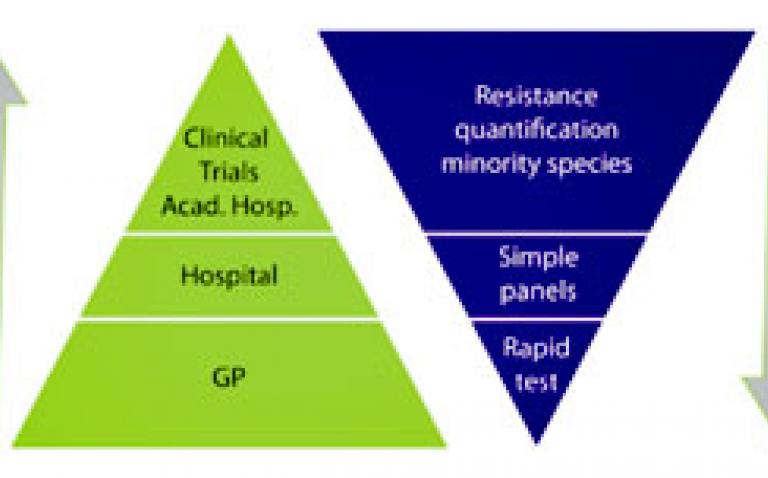
In clinical trials with antivirals that directly inhibit virus production, quantitative culture may give information on the duration of infectious virus shedding and the ability to reduce transmissibility. Quantitative culture requires high-level expertise and is routinely offered by Viroclinics in clinical trials for new antiiviral influenza drugs. The various levels of required influenza diagnostics applied in different clinical settings are illustrated in this figure above.
Viroclinics’ broad and deep expertise
Interpretation and technical performance of diagnostic tests for influenza require a high level of expertise and Viroclinics would like to share its expertise in this field with other labs. With the information shared in the MLO article, Viroclinics intends to serve the biopharmaceutical industry for the development of antivirals and vaccines with state-of-the-art diagnostics, custom-made models in (pre)clinical drug testing and clinical trial operations services.
Link to the online article in MLO: