The US Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee has voted favourably by a majority, Yes: 13, No: 1, that the benefits of the WATCHMAN Left Atrial Appendage Closure device outweigh the risks. The FDA Panel was further asked if there is reasonable assurance that the device is safe, the Panel voted Yes: 13, No: 1. On the question of reasonable assurance of efficacy, the Panel voted Yes: 13, No: 1. The FDA will take into account the Panel’s vote in its decision on approval of the WATCHMAN device. The company expects a decision from the FDA in the first half of 2014.
“We are pleased with the outcome of today’s Panel, which represents an important milestone toward making this innovative technology available to patients with AF at higher risk for stroke who need an alternative to long-term warfarin therapy,” said Kenneth Stein, MD, Chief Medical Officer, Cardiac Rhythm Management, Boston Scientific. “We appreciate the opportunity to present our comprehensive data supporting the WATCHMAN technology and look forward to continuing discussions with the FDA regarding the Panel’s comments.”
The vote of the committee followed a review of clinical data from two randomised control trials, PROTECT AF and PREVAIL, as well as from the CAP (Continued Access Protocol) registry. WATCHMAN is the most studied left atrial appendage closure device and the only one with long-term clinical data from 2,000 patients and with almost 4,900 patient-years of follow-up in clinical trials. The WATCHMAN device received CE Mark in 2005. In the United States, WATCHMAN is an investigational device, limited to investigational use and not available for sale.
About atrial fibrillation and stroke
Atrial fibrillation (AF) is an irregular heartbeat that can lead to blood clots, stroke, heart failure and other heart-related complications. AF is the most common cardiac arrhythmia, currently affecting more than five million Americans.(1) Patients with AF have a five-fold increased risk of stroke due to blood stagnating from the improperly beating atrium and the resulting blood clot formation.(2) Twenty percent of all strokes occur in patients with AF.(3) Stroke is more severe for patients with AF, as they have a seventy percent chance of death or permanent disability.(2) The most common treatment for stroke prevention in patients with AF is blood-thinning warfarin therapy. Despite its proven efficacy, long-term warfarin therapy is not well-tolerated by some patients and carries a significant risk for bleeding complications.
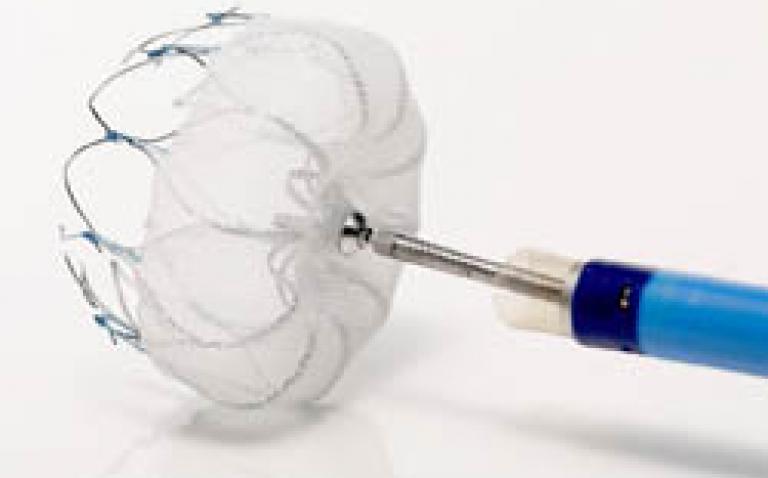
About the WATCHMAN device
The WATCHMAN device is a catheter-delivered heart implant designed to close the left atrial appendage (LAA) in order to prevent the migration of blood clots from the LAA, and thus, reduce the incidence of stroke and systemic embolism for higher risk patients with non-valvular AF.
The LAA is a thin, sack-like appendix arising from the heart and is believed to be the source of a majority of stroke-causing blood clots in people with AF.(4) The WATCHMAN device is commercially available in more than 55 countries, and over 7,000 implants have been performed worldwide. The device was developed by Atritech, which Boston Scientific acquired in March 2011. Images of the WATCHMAN device are available at
References
- Colilla et al., Am J Cardiol. 2013; 112:1142-1147
- Holmes DR, Seminars in Neurology 2010; 30:528–536
- Hart RG, Halperin JL., Ann Intern Med. 1999; 131:688–695
- Blackshear J. and Odell J., Annals of Thoracic Surgery. 1996; 61:755-759