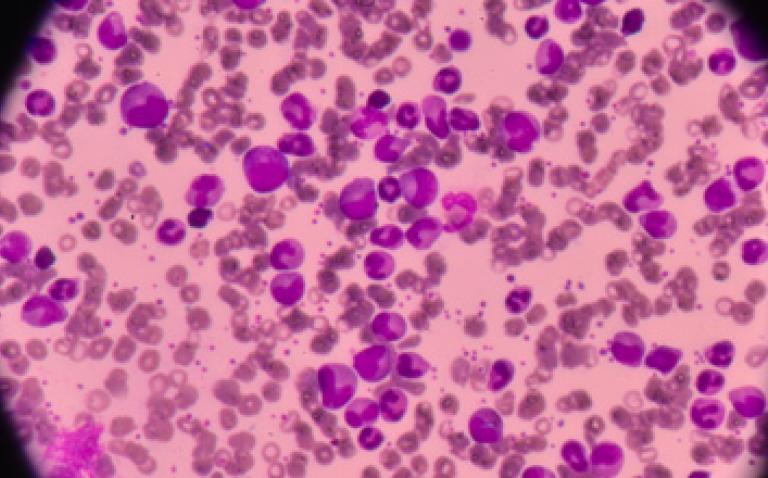
The European Commission has approved Vyxeos 44 mg/100mg powder for concentrate for solution for infusion for the treatment of adults with newly-diagnosed, therapy-related acute myeloid leukaemia (t-AML) or AML with myelodysplasia-related changes, manufacturer Jazz Pharmaceuticals has announced.
Vyxeos is an advanced liposomal formulation that delivers a synergistic molar ratio of daunorubicin and cytarabine, Jazz Pharmaceuticals said.
The EC approval extends to all European member states as well as Iceland, Norway sand Liechtenstein.
Jazz Pharmaceuticals president and chief operating officer Daniel Swisher said: “Vyxeos is the first chemotherapy to demonstrate an overall survival advantage versus the standard of care in a Phase 3 study of older adult patients with newly diagnosed therapy- related AML or AML with myelodysplasia-related changes.Jazz is committed to making Vyxeos available to patients in the EUand we will now pursue rolling launches of Vyxeos across the European Union on a country-by-country basis as pricing and reimbursement decisions are made.”