Infection following prosthetic joint infection (PJI) is a recognised surgical complication. However, it has serious consequences requiring both surgical and antimicrobial management. Patients with rheumatoid arthritis (RA) are both more likely to have joint replacement due to abnormal anatomy and be immunosuppressed due to the underlying disease and immunomodulatory therapies. These factors make them more at risk as a population for post- operative infection.
Epidemiology
Due to progressive joint destruction, patients with RA commonly have to undergo joint replacement. It is estimated that 24% of patients with RA will eventually require their first major joint replacement at 16–20 years after diagnosis.1 Of those who have a total hip or knee replacement 5–7% have RA compared to 1% in the general population.2 There is an increased risk of PJI due to the underlying RA which can be 2.5-times that of a person with osteoarthritis.2
Pathogenesis
In the general population, infection in PJI can occur from direct seeding peri- or post-operatively or haematogenous spread or recurrence of existing infection from previous prosthesis. The prosthetic material allows the bacteria to form a protective glycocalyx layer or biofilm. This means the bacterial colonies deep within this are protected from host defences and antimicrobials. There may also be poor wound healing and poor host defences due to the underlying inflammatory arthritis in patients with RA. This, coupled with immunomodulatory therapy, makes a favourable environment for infection.
Investigation
According to the International consensus meeting in 2013 on peri-prosthetic joint infections, the diagnostic criteria for PJI is as follows.3
Major
- Two positive peri-prosthetic cultures with phenotypically identical organisms
- Sinus tract communicating with the joint.
Minor
- Raised C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR)
- Raised synovial fluid white blood count on leucocyte esterase test strip
- Raised synovial fluid polymorphonuclear neutrophil percentage
- Positive histological analysis of peri-prosthetic tissue
- A single positive culture.
A patient is required to have one major or three minors to be diagnosed. However, in a patient with RA, there are limitations to these criteria.
Initial investigation of prosthetic joint infection in patients with RA is similar to other patients. A careful history and examination should be taken to ellicit:
A) Local features of infection joint swelling, pain, wound breakdown and sinus tract
B) Past medical and surgical history, including previous revisions, microbiology and previous antimicrobial treatment, previous limb cellulitis and other prosthetic material
C) Medication and drug allergy.
There should also be clear documentation on the use of immunosuppressants, acute prescriptions of corticosteroids and infusion therapy that may not be listed on patients’ regular medications as this can affect the immune system up to 12 months after administration.
Full blood count, urea and electrolytes, CRP, ESR and liver function tests should also be part of the standard work up. However, in patients who have RA it might be difficult to differentiate between raised inflammatory markers due to infection or underlying inflammatory arthritis flare. CRP may also be falsely low due to use of immunomodulatory therapies. White blood cells can be elevated due to corticosteroid use.
Other biomarkers specific for bacterial infection, for example, procalcitonin, interleukin-6 (IL-6) and tumour necrosis factor, are in development. A prospective study looking at the sensitivity and specificity of these in prosthetic joint infection identified a combination of IL-6 and CRP as the most useful and that procalcitonin was the most specific.4
There has also been increasing interest in using joint fluid biomarkers to aid in the diagnosis of PJI (for example, CRP, adenosine deaminase (ADA), alpha-2-macroglobulin) which show some promise increasing the positive predictive value in diagnosing PJI vs aseptic loosening.5 Leucoesterase dip stix are easy to use and commercially available but again could also be positive due to underlying RA rather than infection. However none of these biomarkers have been evaluated specifically in patients with inflammatory arthritis with PJI or in patients on immunomodulatory agents. This remains an area for potential research.
With regards to radiology, plain X-rays may be useful for operative planning and for demonstration of loosening of the prosthesis but have a low specificity and sensitivity in differentiating septic and aseptic osteolysis. Plain CT may be used in determining periosteal reaction or soft tissue accumulation. MRI is the most sensitive modality for detection of pus but is limited by its cost and the time the scan takes. Both MRI and CT can be limited by metal artefacts in patients with prosthetic joints. Nuclear medicine scans and FDG PET are useful tools for detection of infection. However, in a patient with RA, there may be difficulties determining the difference between inflammatory arthritis and an infected joint.
The most common pathogens in PJI are Staphylococcus aureus and Coagulase-negative Staphylococcus. However, other bacteria (for example, Streptococcus, Enterococci, Diptheroids, Gram negatives and Anaerobes) can also frequently cause infection. Moreover, PJI infections are often polymicrobial, which can make antimicrobial therapy challenging. The incidence of polymicrobial infection in RA in one cohort was 15%.6 Pathogens causing infection are broadly similar in patients with and without RA – perhaps with a higher incidence of Staphylococcus aureus than the general population.6,7
Obtaining a microbiological diagnosis is key to securing optimal treatment. It is recommended that a deep joint aspirate is collected aseptically – ideally in theatre by a specialist to prevent contamination and further infection of the joint. It should be Gram stained and cultured. If the patient is febrile blood cultures should be taken. Ideally cultures should be collected before commencing systemic antibiotics to increase culture sensitivity.
If atypical organisms are suspected, for example where a history suggests tuberculosis, the microbiology lab must be alerted so that appropriate culture methods can be used and laboratory safety precautions taken.
Mycobacterial infection should be investigated and managed by an experienced physician, looking for a history of exposure or previous infection and examination and imaging for disseminated infection. Fluid should be sent for mycobacterial culture and Mycobacterial polymerase chain reaction (PCR).
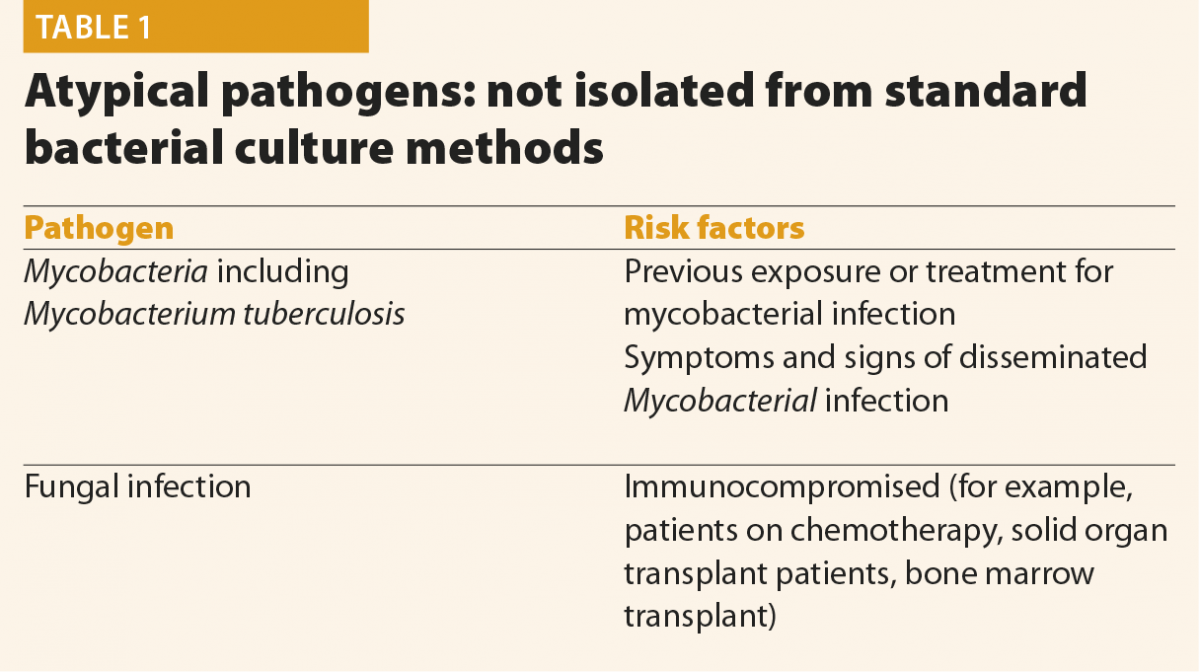
If the patient is felt to be at risk of fungal infection, fluid and blood cultures should be sent with fluid being cultured on fungal selective agar. Blood markers such as beta glucan and Aspergillus antigen, if available locally, should be sent. These cases should be discussed with local Infection specialists to ensure correct specimens are sent.
In the setting of culture-negative PJI, that is, one where no pathogen has been isolated, the patient should be evaluated for the likelihood for a less typical pathogen. There should also be discussion with an infection specialist about empirical antimicrobial therapy, which is usually directed at Staphylococcus species. Tissue samples can also be sent for 16S and 18S PCR analysis to improve sensitivity of detection of bacteria and fungi respectively. This may be useful if the sample was taken on antimicrobials. The limitations with PCR can be availability, possible contamination (and therefore detection of organism that is not the infecting pathogen) and the lack of antibiogram data.
Histological analysis can be used for the diagnosis of PJI. A systemic review of intra-operative frozen sections demonstrated it was a very good predictor of culture positive joint infection and moderate at predicting culture negative cases based on the presence or not of acute inflammation, that is, >5 PMN in at least five separate high powered fields.8 Stains can also be performed for fungi or mycobacterial infection. However there was no subgroup analysis of patients with inflammatory arthritis and whether this affects histological diagnosis. The presence of acute inflammation may not be present in less virulent organisms, for example, Cutibacterium acnes.
Management
The management of PJI in general comprises both medical and surgical options with a multidisciplinary team including orthopaedic and plastic surgeons, infection specialists and rheumatologists. Conventional surgical options for the infected joint replacement are retention and debridement, one or two stage revision procedures, resection of arthrodesis or, as a last resort, amputation.
During surgery, multiple (five to six) deep samples should be taken for Gram stain and culture, each with fresh sterile instruments and without passing through a sinus in order to prevent contamination.
Sonicade and bead milling are newer intraoperative techniques to increase culture sensitivity.
Sonicade involves placing bone tissue in sterile water then passing ultrasound waves through this media. It has been shown to increase the sensitivity of culture and also PCR detection.9
Bead mill processing involves grinding of bone fragments and then processing in blood culture and agar which appear to decrease turnaround times.
There have been several studies looking at the outcomes in surgical management in patients with RA.6,7 One retrospective study looking at 246 episodes of PIJ in patients with RA showed that as per the general population, a two-stage procedure was associated with the best outcome and highlighted that not using antimicrobial impregnated cement was associated with risk of re-infection.7 For all the reasons mentioned, the risk of re-infection of the prosthetic joint is unsurprisingly higher in patients with RA-up to 25% compared with 5% in the general population in both of these studies even with a two-stage procedure. Both studies showed contrasting results with regards to immunosuppressive therapies and the influence on outcomes, leaving this an area for future research. Ultimately any non-randomised control study looking at surgical outcomes in this field is limited by the fact that patients undergoing a two-stage procedure are more likely to have favourable joint anatomy and have fewer comorbidities. This reflects the fact that the final operative management rests with the surgical team.
With regards to medical or antimicrobial therapy of PJI infections this is highly individualised by the micro-organisms and their susceptibilities. Every patient with PJI should be reviewed by an infection specialist. The IDSA10 recommends two to six weeks of pathogen specific therapy in combination with oral rifampicin in susceptible isolates with follow-on oral therapy for three months in total hip replacement and six months in total knee replacement. It also states that IV therapy should go on for four to six weeks if rifampicin cannot be used, but evidence is lacking and this is not our local practice. Lifelong suppressive antimicrobials may be considered if removal or further revisions cannot be performed. With regards to a patient with RA and PJI, the specific medical management might also be affected by drug interactions with immunosuppressive therapy and decisions must be made within a skilled specialist multidisciplinary team.
Other important medical management considerations in PJI infection in RA is the discontinuation of immunosuppressive therapy in elective patients admitted for revision arthroplasty. This should be performed in conjunction with a rheumatologist, especially when considering stopping long-term corticosteroids due to the risk of developing acute adrenal insufficiency. The international consensus meeting on peri-prosthetic joint infections defined timings based on half-lives for stopping immunomodulatory therapy for elective joint replacement. This remains an area for research in patients with PJI. The decision to stop, reduce or continue with the current immunosuppression will require an individualised risk vs benefit analysis.
As with diagnosis, follow up and response to medical and surgical of PJI in RA can be challenging. The joint might be inflamed and biomarkers may be persistently raised due to the patients underlying inflammatory condition rather than persistent infection.
Conclusions
PJI infection in RA remains more common than in the general population and is challenging to diagnose and manage. Treatment should be individualised under a multidisciplinary team in a specialised centre. Areas of future research to provide a better evidence base include the effect of RA and immunomodulatory therapies on the newer specific bacterial biomarkers and the effect of immunosuppressive therapies on recovery from PJI infection.
References
1 Kapetnovic MC et al. Orthopaedic surgery in patients with rheumatoid arthritis over 20 years: prevalence and predictive factors of large joint replacement. Ann Rheumatol Dis 2008:67;1412–16.
2 Berbari EF et al. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis 1998;27:1247–54.
3 Parvizi J at al. Definition of periprosthetic joint infection. J Arthoplasty 2014;29(7):1331.
4 Bottner F et al. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg 2007;89:94–9.
5 Sousa R et al. Improving the accuracy of synovial fluid analysis in the diagnosis of prosthetic joint infection with simple and inexpensive biomarkers: C-reactive protein and adenosine deaminase. Bone Joint J 2017;77:351–77.
6 Berbari EF et al. Outcome of prosthetic joint infection in patients with rheumatoid arthritis: the impact of medical and surgical therapy in 200 episodes. Clin Infect Dis 2006;42:216–23.
7 Hsieh PH, Huang KC, Shih HN. Prosthetic joint infection in patients with rheumatoid arthritis: an outcome analysis compared with controls. PLoS One 2013;8:e71666.
8 Tsaras G et al. Utility of intraoperative frozen section histopathology in the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am 2012;94:1700–11.
9 Trampez A et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med 2007;357:654–63.
10 Osmon DR et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013;56:1–10.










