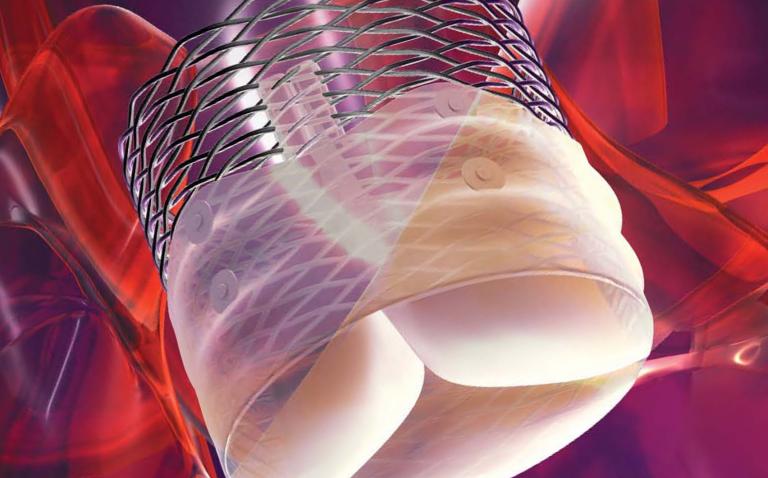
Boston Scientific Corporation (NYSE: BSX) has received the CE Mark for the Lotus™ Valve System, the company’s advanced transcatheter aortic valve replacement (TAVR) technology. This key approval offers a unique and effective new treatment alternative for patients with severe aortic stenosis at high risk with surgical valve replacement.
The Lotus Valve System is immediately available to select centres in Europe, with commercial site expansion accelerating as physicians and centres become fully trained.
The Lotus Valve System is designed to provide physicians increased control during implantation and to help provide a more precise, predictable procedure. It is the only aortic valve device that can be assessed in its final position prior to release, while maintaining the ability for the physician to reposition or fully resheath and retrieve the valve. The Lotus Valve System also incorporates a unique Adaptive Seal™ technology designed to minimise aortic regurgitation (leaking), a proven predictor of mortality.
“The ability to initially position the Lotus Valve precisely and, if needed, to easily reposition or fully retrieve the valve provides the operator with remarkable control,” said Professor Ian Meredith, director of MonashHeart at Monash Medical Centre in Melbourne, Australia, and principal investigator of the REPRISE II trial. “Combined with early and often complete elimination of aortic regurgitation as observed in REPRISE II, the unique features of the Lotus Valve technology represent a significant step forward in the percutaneous treatment of eligible patients with severe symptomatic aortic stenosis.”
Data presented at the PCR London Valves course in September demonstrated the Lotus Valve System met the primary performance endpoint for the first 60-patient cohort and was implanted successfully in all patients (60/60) with no cases of severe paravalvular regurgitation. In 76.1% of patients there was no corelab adjudicated paravalvular regurgitation at six months.
“Results from the REPRISE II trial highlight the promise behind the Lotus Valve System, especially related to avoiding moderate or severe paravalvular leaks,” said Dr Nicolas M Van Mieghem, MD, Erasmus Medical Center in Rotterdam, The Netherlands. “In addition to providing a new treatment option for TAVR, the Lotus Valve has the potential to improve clinical outcomes by minimising paravalvular leaks.”
“The Lotus Valve System offers patients a new, effective treatment option and provides physicians unmatched positioning and placement capabilities,” said Tom Fleming, vice president and general manager, Structural Heart, Boston Scientific. “It’s the culmination of a decade of research and development and demonstrates our commitment to innovations that make a difference in the lives of patients.”
The Lotus Valve System comes pre-loaded on a transfemoral delivery system which is inserted through a small incision in the leg. Available in a 23mm and 27mm size, the Lotus Valve System can treat patients with aortic annulus sizes from 20mm to 27mm. The Lotus Valve System is an investigational device in the US and Japan and is not available for sale in those countries.