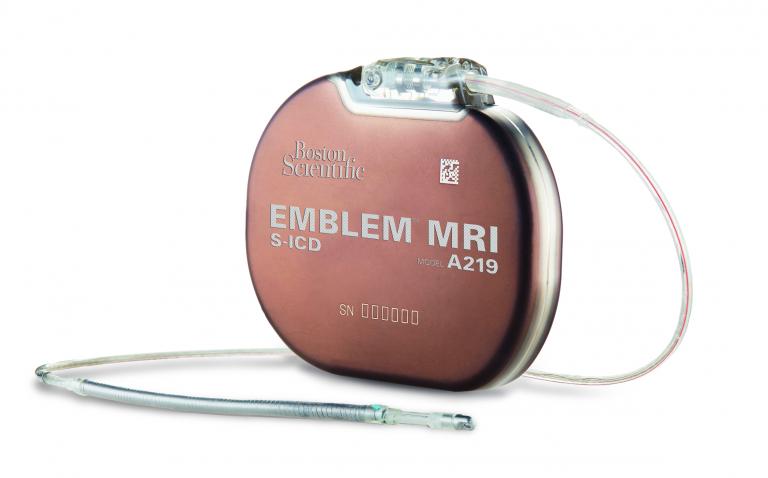
Boston Scientific has received CE Mark approval for the new EMBLEM™ MRI Subcutaneous Implantable Defibrillator (S-ICD) System, as well as magnetic resonance (MR) conditional labelling for all previously implanted EMBLEM S-ICD Systems.
The EMBLEM S-ICD Systems are treatment options for patients at risk of sudden cardiac arrest (SCA) that leave the heart and vasculature untouched, reducing the risk of complications associated with transvenous implantable cardioverter-defibrillators (TV-ICDs). Initial market release of the new EMBLEM MRI S-ICD System has begun in a small number of European centres with a broad European launch scheduled for early this summer.
In Europe, the EMBLEM MRI S-ICD System joins the growing family of ImageReady™ MR-conditional devices, all of which are labelled safe for use in a magnetic resonance image setting when conditions of use are met. Patients receiving the EMBLEM MRI S-ICD System as well as patients who previously were implanted with an EMBLEM S-ICD System are now able to undergo full-body MR scans safely in 1.5 Tesla environments when conditions of use are met.
“These approvals give reassurance to physicians and their patients that they have access to any future MR scan needs, and underscores the Boston Scientific commitment to gain MR-conditional labelling on high-voltage devices that are being implanted today,” said Kenneth Stein, MD, chief medical officer, Rhythm Management, Boston Scientific. “Further, the EMBLEM S-ICD System is a compelling treatment option for the majority of ICD-indicated patients that provides protection from cardiac arrest without invading the heart and blood vessels.”
The EMBLEM MRI S-ICD System also includes two new features, SMART Pass technology and Atrial Fibrillation (AF) Monitor™. The SMART Pass technology will help ensure patients receive therapy from the device only when necessary by enhancing the INSIGHT™ Algorithm, which identifies and classifies a heart rhythm for effective arrhythmia treatment. This novel feature will also be added to previously implanted EMBLEM S-ICD Systems through a software update. The AF Monitor feature of the EMBLEM MRI S-ICD System is a new detection tool designed to alert physicians after the identification of AF so they can make more informed treatment decisions for their patients.
The company is actively pursuing US Food and Drug Administration (FDA) approval of the EMBLEM MRI S-ICD System as well as MR-conditional labelling for previously implanted EMBLEM S-ICD Systems. Additionally, the global ENABLE MRI study, initiated earlier this year, is intended to support FDA approval for MR-conditional labelling across the company’s currently approved ICD and cardiac resynchronisation therapy systems.
For more information on the EMBLEM S-ICD Systems visit www.sicdsystem.com.
In the US, the EMBLEM MRI S-ICD System is not available for sale.