In December 2018, the National Institute for Health and Care Excellence (NICE) published its long-awaited update to the 2010 guideline on chronic obstructive pulmonary disease (COPD) in over 16-year-olds. A long time had elapsed between the update and previous guideline, and other guidance, such as that produced (and updated more frequently) by the Global initiative for chronic Obstructive Lung Disease (GOLD), has been useful in the interim to individualise treatment for patients. This article will outline the significant changes in the NICE guidance.
COPD is a progressive long-term condition and leading cause of death and disability; it has an estimated cost to the National Health Service over £800 million pounds per year. The mortality rate in England is roughly 23,000 deaths each year (around one person every 20 minutes).1
COPD is associated with current or history of smoking and/or biomass fuel/noxious particle exposure and usually affecting people over the age of 35 years (and often diagnosed in their 50’s). The ageing population is living for longer, often with a poorer quality of life, which itself presents a challenge to the healthcare system. Current generations may have started smoking from a younger age than previous, resulting in earlier onset of poor health. Prevalence is associated with geographical levels of deprivation and is increasing; many millions remain undiagnosed and by 2020, COPD will be the third leading cause of death globally.1,2
COPD is characterised by breathlessness and cough. Patients will typically experience exacerbations (some patients more so) which negatively impacts disease progression, rates of hospitalisation and readmission and health status. The number of exacerbations in the year prior is the strongest predictor of a patient’s future exacerbation frequency.3 The rate of lung function decline is faster in the earlier stages of the disease, which can be modified by treatment.1
The new guideline specifically acknowledges the need for a secure diagnosis, made using signs and symptoms and confirmed through spirometry by appropriately trained healthcare workers (who have up to date skills and are competent in interpreting results). This includes noting exacerbation history, excluding conditions such as asthma/cancer and consideration of alternative diagnoses such as alpha-1 antitrypsin disease. Diagnosis should also be considered in symptomatic individuals with normal spirometry. Oral steroids should not be used for reversibility testing or to predict response to inhaled corticosteroid (ICS) in individuals.2
Key opinion leaders and emerging research suggest that there are disease phenotypes (frequent exacerbator or persistent symptomatic patient).3 However, the place of inhaled triple therapy (where ‘triple therapy’ refers to use of long-acting beta agonist (LABA), long-acting anti-muscarinic agent (LAMA) and ICS together), asthma–COPD overlap management, role of eosinophils3 and role of macrolide antibiotics in disease management are also important considerations (which the guideline attempts to address) but require longer term data to further inform future practice. Updates in this guideline have been made on the following:7
- Investigations, including incidental findings on CT scans – primary care review, advice for patients to return if respiratory symptoms appear, offering smoking cessation and discussing the potential risk of lung cancer
- Prognosis – to avoid use of a multidimensional index such as BODE to assess prognosis
- Inhaled therapies – to discuss risk of pneumonia and ICS use with patients. Minimising the number and types of inhalers patients use and ensuring they are trained on use
- Oral phosphodiesterase 4 inhibitors are mentioned in line with the associated 2017 technology appraisal
- Prophylactic antibiotics – recommendations made on (unlicensed) use of azithromycin
- Oxygen therapy – eligibility of patients for and risks of prescription (including short burst and ambulatory use) and consideration for use in pulmonary hypertension (noting this is not a treatment for breathlessness and not effective for isolated nocturnal hypoxaemia caused by COPD)
- Managing pulmonary hypertension and cor pulmonale – advice on optimised therapies
- Lung volume reduction (LVR) surgery and procedures – updated advice to increase uptake/access
- Self-management/exacerbation plans – developed in collaboration with patients and carers.
These supplement the existing recommendations on:
- diagnosing COPD using symptoms, spirometry and other tests
- managing stable COPD using nebulisers, oral therapy and pulmonary rehabilitation
- multidisciplinary management of stable COPD, including physiotherapy, occupational therapy, nutrition and palliative care
- managing exacerbations of COPD in primary care and in hospital.
NICE has produced a visual summary alongside the guidance, covering non-pharmacological management and use of inhaled therapies (Figure 1). They also published concurrent antimicrobial prescribing guidance for acute exacerbations of COPD (December 2018).
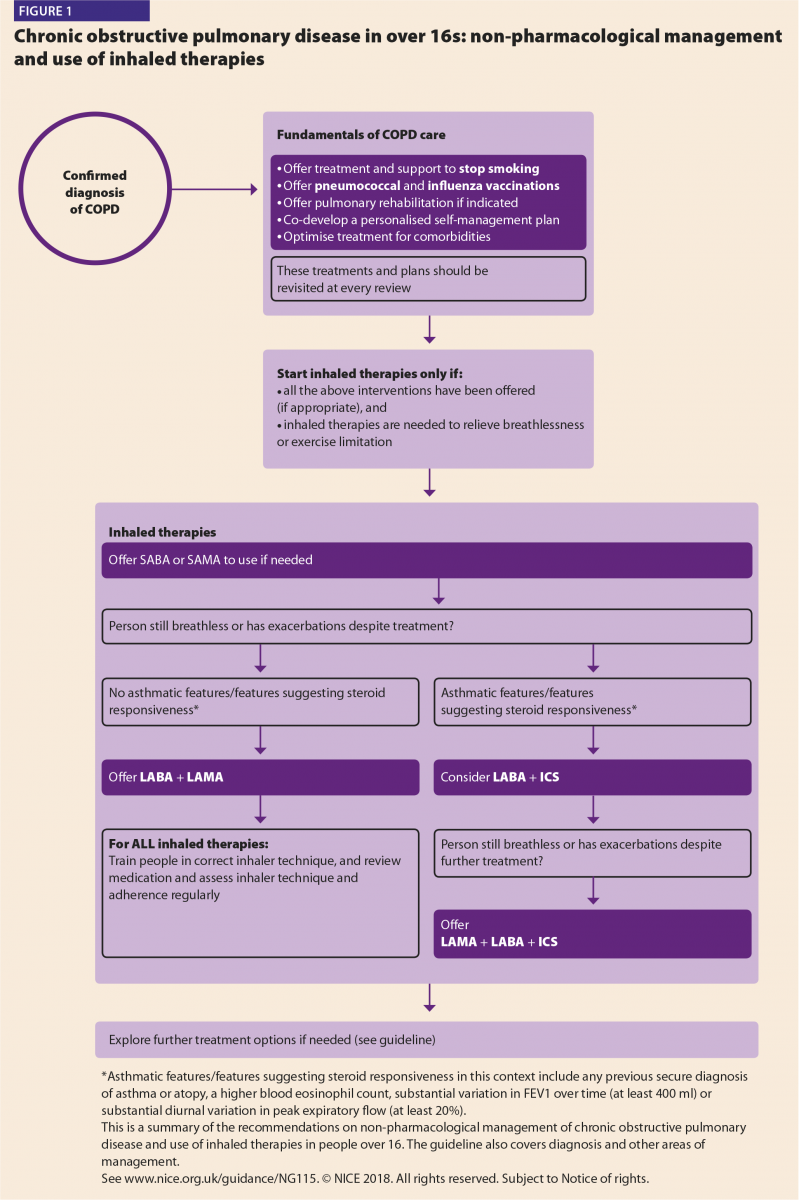
The 2010 guidance inevitably steered patients towards triple therapy (with LABA, LAMA and ICS) with a greater emphasis on FEV1 (forced expiratory volume in one second) value to guide this. Early therapy started with a short-acting bronchodilator progressing to a single long-acting bronchodilator (evidence subsequently showed superiority of LAMA over LABA use first line, especially in moderate–severe patients due to lower incidence of exacerbations and adverse effects with LAMA);4,5 stepping up to dual long acting agent and then addition of an ICS (a suggestion which was based on expert opinion).
It is now apparent that as a result, some patients were treated with ICS who may not have warranted it. The new guidance still initiates inhaled therapy with a short-acting bronchodilator but progresses to long-acting
dual therapy.
The new guidance does not rely on FEV1 to adjust therapy. One key change, stepping up after short acting bronchodilator, is the initiation of dual bronchodilator (LABA+LAMA) in preference to a single long acting agent. For patients exhibiting asthmatic features, the dual therapy would be initiated with ICS and LABA rather than LABA/LAMA [‘Where asthmatic features/features suggesting steroid responsiveness in this context include any previous secure diagnosis of asthma or atopy, a higher blood eosinophil count, substantial variation in FEV1 over time (at least 400ml) or substantial diurnal variation in peak expiratory flow (at least 20%)’].2
Patients using long-acting therapy outside of the December 2018 recommendations can continue on current treatment and change after review with a healthcare professional (HCP), when both agree is appropriate.
NICE is undertaking a separate review on the role of triple therapy in COPD (which was out of scope of the initial guidance update). At time of writing this article, the draft is undergoing public consultation and final guidance is scheduled to be published in Summer 2019. The draft 2019 guidance showed the proposed place of triple therapy in patients who remain breathless +/- exacerbate despite all other interventions being optimised (noting consideration of a three-month trial in those without asthmatic features, reverting if ineffective).6
Management
Management of COPD can be broadly divided into two areas – non-pharmacological and pharmacological.
Non-pharmacological
These measures should be optimised alongside any pharmacological intervention and before escalation of therapy:
- Pulmonary rehabilitation, especially encouraging physical activity to minimise muscle loss/deconditioning (which increases mortality)
- Optimised nutrition – dietetic input and supplementation where applicable
- Social/occupational therapy input to retain independence and enable activities of daily living
- Psychological support for anxiety and depression +/- pharmacological treatment as necessary7,8
- Airways breathing control techniques to aid energy conservation, management of panic/anxiety
- Use of a hand-held fan may provide relief of breathlessness.9
Pharmacological
- Inhaled therapies initiated appropriate to the stage of disease (Note any patients exhibiting asthmatic features should have an ICS in combination with a LABA)
- Smoking cessation can benefit most diseases and should be offered as a ‘treatment’.10 Studies in the UK and overseas have demonstrated that behavioural support plus access to pharmacotherapy is effective in helping smokers to quit. Regular meetings (group or one to one) with a trained adviser using structured, withdrawal-oriented behavioural therapy combined with smoking cessation medications such as nicotine replacement therapy (NRT), bupropion or varenicline11,12
- Vaccination – Annual flu and five-yearly pneumococcal vaccinations to minimise incidence of respiratory infections2,13
- Mucolytic drug trial (anecdotally 4–6 weeks) could be considered for chronic productive cough but should be stopped if not beneficial and used with caution in those with peptic ulceration history2,14
- Oral theophyllines may be beneficial in patients who remain breathless despite trialling inhaled therapy or for those who cannot use inhaled therapy (noting potential for interactions and requirement for plasma monitoring)
- Roflumilast (phosphodiesterase IV inhibitor) can be used as adjunctive therapy in patients with severe COPD associated with chronic bronchitis and frequent exacerbations
- Oxygen – assessment for long term/ambulatory oxygen therapy in non-smokers with more severe chronic hypoxaemia (assessing risks to patient and others of prescribing). Supplemental oxygen should be used >15 hours a day; long-term oxygen therapy is not effective for isolated nocturnal hypoxaemia caused by COPD
- Prophylactic azithromycin may reduce exacerbations in non-smokers who have had all other interventions optimised yet continue to have >1 of the following: frequent exacerbations (typically >4 per year) with sputum production, prolonged exacerbations with sputum production or exacerbations resulting in hospitalisation. Note this is unlicensed therapy requiring monitoring before and during therapy
- ‘As required’, low dose lorazepam (0.5mg) and morphine sulphate liquid (2.5mg) for anxiety and breathlessness respectively, are useful in more end stage disease where patients may suffer panic attacks.2,14,15
Other considerations
- Medication adherence – understanding barriers to this and medicines optimisation incorporating the patient’s perceptions and practicalities of living with the disease and how this impacts medicine-taking behaviour16,17
- Inhalers – device best suited to patient, appropriate for inspiratory flow, coordination, dexterity etc – technique checked and corrected by appropriately trained HCPs, ensuring maximal lung deposition (further assisted by use of a spacer device with metered dose inhalers (MDIs)). Use of combination inhalers to minimise polypharmacy2,16,17
- Empowerment and education for patients and HCPs (including co-created self-management plans, strategies for managing exacerbations and escalating therapy) safety-netting/signposting to support groups, digital solutions and further reliable information sources for example, from the British Lung Foundation
- Air pollution (indoor and outdoor) – impact to patient
- Environmental impact – waste/recycling, carbon footprint and ozone damage by hydrofluoroalkane propellants in MDIs
- Passive smoke – other members of the household who smoke might impact on the patient’s health (including eligibility for oxygen) and could benefit from smoking cessation interventions
- Multi-morbidity and frailty – effect of this on functionality of the patient, including increased likelihood of polypharmacy and potential non-adherence to therapies18
- Palliation – early discussions with patient and referral for palliative input.19
Quality standards and outcomes
NICE quality standards are a concise set of prioritised statements designed to drive measurable improvements in the three dimensions of quality – patient safety, patient experience and clinical effectiveness – for a particular area of health or care. They are derived from highquality guidance from, for example, NICE or other sources accredited by NICE. The COPD quality standard (last updated in 2016), in conjunction with the guidance, should contribute to the improvements outlined in the following three outcomes frameworks published by the Department of Health:20
NHS Outcomes Framework 2015–16
Reducing premature mortality, enhancing quality of life for people with long term conditions, helping people to recover from episodes of ill health or following injury and ensuring that people have a positive experience of care.
Public Health Outcomes Framework 2013–16
Health improvement/healthcare public health and preventing premature mortality.
Adult Social Care Outcomes Framework 2015–16
Delaying and reducing the need for care and support.
The quality standard is expected to contribute to improvements in the following outcomes:
- COPD diagnosis
- morbidity
- mortality
- acute exacerbations
- hospital admissions
- A&E attendance
- quality of life
- change in breathlessness
- exercise capacity
- inappropriate non-invasive ventilation.
Guideline implications
The new guideline encourages holistic treatment and will impact current practice; an increase in numbers of counselling/consultation episodes (including time taken) may result. Professionals are expected to be qualified to provide high-quality spirometry and interpretation for appropriate diagnosis and earlier management. Prescribing will change, replacing single long-acting inhaled agents with dual (increasing LABA/LAMA prescribing) and reducing inappropriate ambulatory and short-burst oxygen prescribing. Smoking cessation intervention should increase.
Close communication will be required across sectors to ensure continued prescribing, monitoring and review of medications, especially for macrolides and oxygen. There is an expected increase in referrals for LVR interventions. Increased monitoring and pharmacovigilance will be necessary to minimise and manage medication adverse effects. Greater patient empowerment and increased self-management plans are expected (tailoring therapy for maximal benefit and reducing hospitalisation).
Conclusions
The NICE guideline has been long overdue; it conflicts with the most recent 2019 GOLD COPD guidance on prevention, diagnosis and management, which might cause clinicians some confusion as to which guideline to use. GOLD provides pragmatic guidance such as acknowledging the potential role of eosinophils to inform ICS prescribing and is used globally; hence all suggestions might not be applicable/available to UK patients.
NICE has a robust development process, leading to evidence-based recommendations for the most cost-effective interventions to provide maximal societal value benefit. It reiterates the importance of non-pharmacological measures to underpin the overall holistic treatment approach before escalating therapy. It acknowledges the need for appropriately skilled healthcare professionals to be able to diagnose, monitor and review patients with COPD throughout their disease trajectory. This should empower multidisciplinary staff (such as physiotherapists and pharmacists) to expand current roles, enabling patients’ greater access for review, support and consistent messages of disease management.
The new guidance takes a more considered approach to ICS use. Fixed dose triple inhalers are now available and the role of inhaled triple therapy is becoming clearer; further guidance on this is due in Summer 2019. The guidelines encourage more cost effective, responsible prescribing overall including improving medication adherence and a reduction in waste, which can be the basis for further quality improvement work.
The effect of long-term macrolide antibiotic use is unknown, both in terms of safety and clinical effect, there are no studies beyond one year to inform this. There is also concern of increased antimicrobial resistance as a result. It is expected that this intervention will have little cost implication but may reduce exacerbations and associated costs of health resource utilisation. Patients will need to be monitored closely with increased counselling, pharmacovigilance and ‘yellow card’ reporting to the Medicines Health Regulatory Authority (https://yellowcard.mhra.gov.uk/).
The guidance addresses many current clinically relevant issues in the diagnosis and management of patients with COPD but acknowledges that evidence is still lacking or unclear in some areas, leading to recommendations for research.
COPD has no cure but if guidance is applied, in conjunction with the quality standards, premature mortality can be prevented. Patients can have better healthcare experiences, co-create their care decisions for an improved quality of life and palliation. See the full guideline for detailed guidance and recommendations on the above.
References
1 Department of Health. COPD commissioning toolkit, a resource for commissioners. Department of Health, England, August 2012. www.gov.uk/government/uploads/system/uploads/attachment_data/file/212876… (accessed May 2019).
2 National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management NICE guideline [NG115] Published date: December 2018. www.nice.org.uk/guidance/ng115 (accessed May 2019).
3 Global Strategy for Prevention, Diagnosis and Management of COPD. 2019 report – Global initiative for chronic obstructive lung disease. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14N… (accessed May 2019).
4 Chen WC et al. Long-acting beta2-agonists versus long-acting muscarinic antagonists in patients with stable COPD: A systematic review and meta-analysis of randomized controlled trials. Respirology 2017;22:1313–19.
5 Vogelmeier C et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011;364:1093–103.
6 National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2019 update). In development [GID-NG10128]. www.nice.org.uk/guidance/gid-ng10128/documents/draft-guideline (accessed May 2019).
7 National Institute for Health and Care Excellence. Depression in adults: recognition and management Clinical guideline [CG90] Published date: October 2009. Last updated: April 2018. www.nice.org.uk/guidance/cg90 (accessed May 2019).
8 National Institute for Health and Care Excellence. Depression in adults with a chronic physical health problem: recognition and management. Clinical guideline [CG91] Published date: October 2009. www.nice.org.uk/guidance/cg91 (accessed May 2019).
9 Luckett T et al. Contributions of a hand-held fan to self-management of chronic breathlessness. Eur Resp J 2017;50:1700262; DOI: 10.1183/13993003.00262-2017
10 National Institute for Health and Care Excellence. Stop smoking interventions and services NICE guideline [NG92] Published date: March 2018. www.nice.org.uk/guidance/ng92 (accessed May 2019).
11 Bauld L et al. The effectiveness of NHS smoking cessation services: a systematic review. J Publ Health 2009:1–12.
12 Hoogendoorn M et al. Long-term effectiveness and cost effectiveness of smoking cessation interventions in patients with COPD. Thorax 2010;65:711–18.
13 Public Health England. Immunisation against infectious disease — ‘The Green Book’.
14 British National Formulary. Number 76. September 2018 – March 2019. British Medical Journal Group and Royal Pharmaceutical Society (Great Britain).
15 Barnes H et al. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Systematic Review – Intervention Version published: 31 March 2016. www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011008.pub2/full#CD0… (accessed May 2019).
16 National Institute for Health and Care Excellence. Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence Clinical guideline [CG76] Published date: January 2009. www.nice.org.uk/Guidance/CG76 (accessed May 2019).
17 National Institute for Health and Care Excellence. Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes NICE guideline [NG5] Published date: March 2015. www.nice.org.uk/guidance/ng5 (accessed May 2019).
18 National Institute for Health and Care Excellence. Multimorbidity: clinical assessment and management NICE guideline [NG56] Published date: September 2016. www.nice.org.uk/guidance/ng56 (accessed May 2019).
19 National Institute for Health and Care Excellence. End of life care for adults. Quality standard [QS13]. Published date: November 2011. Last updated: March 2017. www.nice.org.uk/guidance/qs13/chapter/List-of-statements (accessed May 2019).
20 National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in adults. Quality standard [QS10]. Published date: July 2011. Last updated: February 2016. www.nice.org.uk/guidance/qs10 (accessed May 2019).










