Carbomer-containing eye gels branded Aacarb, Aacomer and Puroptics have a potential risk of microbial contamination and must be withdrawn from use, the Medicines and Healthcare products Regulatory Agency (MHRA) has warned.
Precautionary safety advice regarding possible infection from the gels has been issued, with healthcare professionals and retailers being advised to withdraw the products. The MHRA is also urging patients to stop using them immediately and return them to their place of purchase.
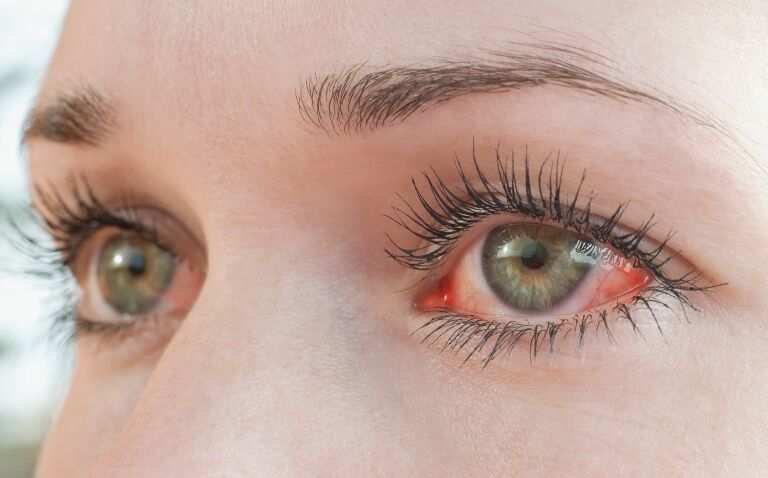
Users of the gels, which are used to treat dry eye, who have symptoms of an eye infection, such as reduced vision and red or painful eyes, have also been advised to contact a healthcare professional and tell them they have been using an eye gel that has been recalled.
Individuals with cystic fibrosis and patients with certain risk factors are at higher risk of adverse effects from Burkholderia cenocepacia – the bacteria which may have caused the microbial contamination.
As a precautionary measure, the UK Health Security Agency (UKHSA) is additionally recommending that all carbomer-containing lubricating eye gels are avoided where possible in individuals with cystic fibrosis (CF), patients being cared for in critical care settings, those awaiting lung transplantation and anyone who is severely immunocompromised.
The Faculty of Intensive Care Medicine ’wishes to draw members’ attention to the protection briefing note from the UKHSA’.
The possible contamination risk was identified as part of an investigation conducted by the UKHSA after a small number of cases of infection were reported. However, investigations are ongoing and are not conclusive, the MHRA said, adding that the risk to the general public is considered to be low.
A novel cluster of Burkholderia cenocepacia involving 20 cases were identified across the UK. Of these cases, 12 (60%) are male, 18 (90%) are adults and two are paediatric patients (age range six weeks to 82 years, median 57 years). A total of 14 (70%) were critical care inpatients, and two patients have cystic fibrosis.
‘We are working very closely with our colleagues at UKHSA and will issue further advice to protect patients and the public, if needed,’ said Alison Cave, MHRA chief safety officer.
UKHSA is also working with NHS England and CF expert stakeholders to issue communications directly to CF patients, who will be directed to their CF Treatment Centres for further clinical advice.
The MHRA is urging healthcare professionals, or anyone who suspects they may have become unwell after using these eye gels, to report it to the agency’s Yellow Card Scheme.
A version of this article was originally published by our sister publication The Pharmacist.