Oraya Therapeutics Inc. and Bridge Design announced that the Oraya IRay® Radiotherapy System has won the silver award in the radiological and electromechanical devices category of the 18th Annual Medical Design Excellence Awards (MDEA).
The winners were announced at a ceremony in New York City at the Jacob Javits Center in conjunction with the MD&M East event.
The MDEA is the medtech industry’s premier design competition committed to searching worldwide for the highest calibre finished medical devices, products, systems, or packaging available on the market. The awards programme celebrates the achievements of the medical device manufacturers, their suppliers, and the many people behind the scenes – engineers, scientists, designers, and clinicians – who are responsible for the cutting-edge products that are savings lives; improving patient healthcare; and transforming medtech – one innovation at a time.
The 2015 MDEA Juror Panel selected 45 exceptional finalists in 10 medical technology product categories. Products were judged based on design and engineering innovation; function and user-related innovation; patient benefits; business benefits; and overall benefit to the healthcare system.
“We, alongside our partner Bridge Design, are honoured to be recognised with this prestigious award,” said Jim Taylor, president and CEO of Oraya Therapeutics. “Our goal in designing and developing the IRay system was to offer ophthalmologists and their patients an innovative, comfortable and non-invasive treatment option for wet AMD. We believe that physician ease-of-use and patient comfort have played a significant role in the rapid adoption of Oraya Therapy in Europe and its continued integration into standard clinical care.”
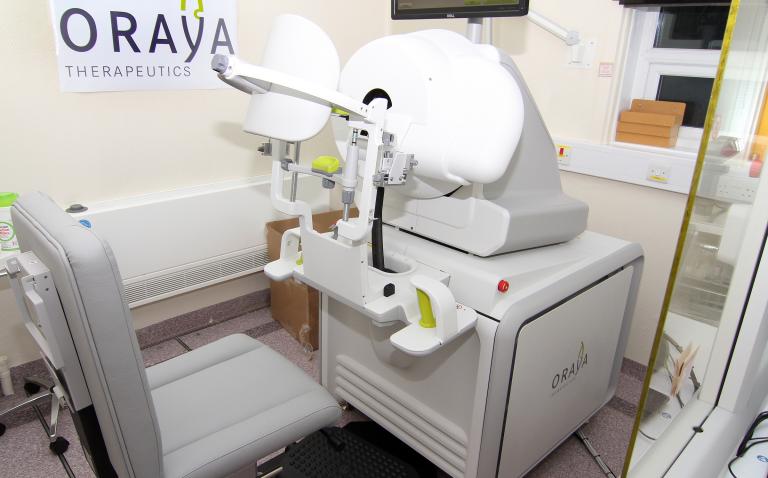
The IRay Radiotherapy System delivers low-voltage X-rays for the treatment of wet age-related macular degeneration (AMD), the leading cause of blindness in those over 65 in the industrialised world. The IRay system’s relatively small footprint is designed for installation in a clinic or hospital, without the need for added room shielding, and to be easy to operate. The key proprietary elements of the system consist of a low-energy X-ray tube, a self-contained automated beam positioning system, Oraya Therapy software, the I-Guide™ eye stabilisation device and an eye tracking system. Combining these elements into a product that would be patient friendly as well as providing reliability and efficiency with patient workflow was critical.
Bridge Design worked in close collaboration with Oraya to design and engineer the IRay system from conception to commercialisation. Extensive ergonomic studies were undertaken to gain a clear understanding of physicians’ and patients’ needs, and to inform the development of the product for an international market. Streamlined, easy operator steps that could reduce procedure time and increase overall patient comfort were also vital to the design.
“The success of any innovative product in the start-up medical world is a combination of the foresight of the founders, the effectiveness of their core technology and the people they pick to help make it a reality,” says Bridge Design’s Principal and Founder Bill Evans. “We are honoured to have been chosen as Oraya’s design partner and glad that our contribution to the usability and customer appeal has helped Oraya achieve rapid adoption. Oraya brought us into their fold, integrating us with their technical and marketing teams. From this teamwork comes the product you see. It is rewarding for our designers to be involved in such vision-maintaining therapy and we wish Oraya well as they expand into new treatment sites.”
Oraya Therapy is available at 11 sites in three countries including the United Kingdom, Germany and Switzerland, and additional sites are in the active planning stages. The IRay system is CE marked in Europe. In the US, the IRay System is an investigational device and is not available for sale.