Bare metal stent (BMS) usage continues to decline. However, there are still conditions when a BMS should be used. In such cases it is important to use the BMS with the lowest target lesion revascularisation (TLR) rate. The REBEL™ stent system, from Boston Scientific, is the newest advance in BMS technology – its advantages include radial strength and low recoil of stent, as TLR rates are generally higher for a BMS compared with a drug-eluting stent.
Ieva Briede
Andrejs Erglis PhD FESC FACC
Pauls Stradins Clinical University Hospital, Latvian Cardiology Center andPauls Stradins Clinical University Hospital, University of Latvia, Latvia
Key words: Stents; imaging; coronary disease
Date received: 20 July 2014
Date accepted: 7 August 2014
For decades, the main limitation of percutaneous treatment of coronary lesions has been restenosis, which affects 10% to 30% of patients. After plain balloon angioplasty, restenosis is mainly due to elastic recoil and negative remodelling of the vessel wall. Intravascular implantation of a metallic stent prevents both problems and results in less restenosis compared with balloon angioplasty alone. However, stent implantation induces a foreign body reaction, leading to excessive neointima formation, which in turn causes in-stent restenosis.1 Drug-eluting stents (DES) reduce neointima formation, but at the expense of a prolonged risk of very late thrombosis due to delayed endothelialisation, earlier and more frequent neoatherosclerosis or both. Data on second-generation DES in combination with prolonged dual antiplatelet therapy are more reassuring on late thrombosis but are currently limited to two to four years follow-up for very late thrombosis.2,3 There has been tremendous progress in stent technology from bare metal stents (BMS) to DES and to biodegradable polymer DES. DES revolutionised the practice of interventional cardiology and coronary revascularisation with significant reductions in the risk of restenosis and consequently the need for repeat revascularisation compared with BMS.4,5 However, despite the introduction of DES more than ten years ago, BMS are still being used, although with varying frequency.6
The use of DES and BMS
The European Society of Cardiology (ESC) guidelines on myocardial revascularisation in 2010 recommended DES usage in almost all patients, but there were some relative clinical contraindications to the use of DES and there was some place for BMS (Table 1).
Recently, Bangalore et al evaluated the trend in DES use across the US from 2001 to 2011.8 Among the 8.1 million coronary procedures, drug-eluting stent use reached a peak in 2005 at 89% in all patients including groups with a low risk of restenosis, high risk of stent thrombosis or bleeding. A steep drop to 66% was noted in 2007 followed by a progressive rise to 73% in 2011, when BMS were used in 26% of all stent implantation. Compared with 2001, the adjusted odds ratio of BMS use dropped 95% by 2011. In 2011, 75% patients with diabetes, 66% patients with a history of bleeding peptic ulcer and 61% patients with a history of atrial fibrillation received DES. BMS was still often used in elderly patients, patients with peptic/gastric ulcers, patients with planned surgery and in those who required long-term anticoagulant therapy.
Although DES usage is increasing in the US, there are some conditions when BMS should be used. In such cases it is important to use the BMS with the lowest target lesion revascularisation (TLR) rate.
BMS studies in the DES era
Many studies have been performed to evaluate the ‘best’ BMS and a number of alternative approaches to DES have been tested. The Genous bio-engineered BMS carries a layer of murine, monoclonal, antihuman CD34 antibody, aimed at capturing circulating endothelial CD34+ progenitor cells, thus possibly increasing the rate of healing. The single-centre pilot TRI-stent adjudication study (TRIAS) did not confirm initial promising results in patients at high risk of coronary restenosis.9 Another single-centre observational study comparing silicon carbide-coated metal stents (PRO-Kinetic) and uncoated BMS (Vision) in 2731 patients was performed in Finland. The PRO-Kinetic stent remained an independent predictor for revascularisation (p=0.002). This study showed that silicon carbide-coated BMS was associated with greater target lesion revascularisation rates at one year compared with the Vision stent.6
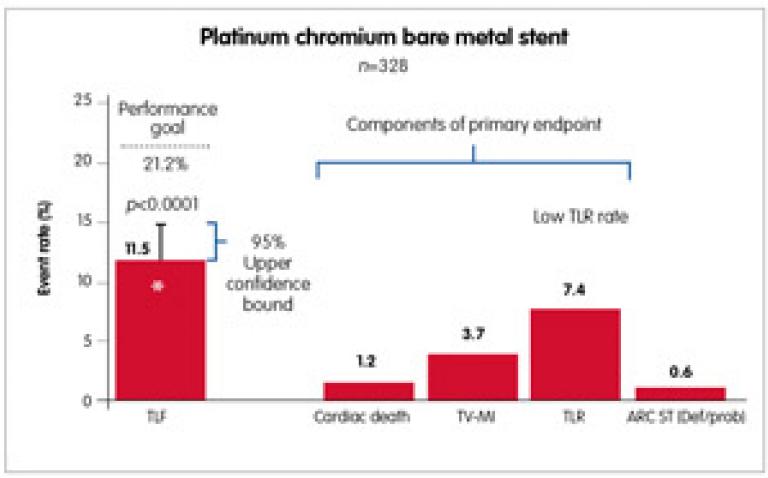
The OMEGA study was the latest prospective, multicentre, single-arm study, which enrolled 328 patients at 37 investigative sites in the US and the EU. Patients received the OMEGA(™) stent (the new REBELTM is the same platinum chromium platform of OMEGA(™), but with innovative customised stent architecture) for the treatment of de novo native coronary artery lesions (≤28mm long; diameter ≥2.25mm to ≤4.50mm). The primary endpoint was nine-month target lesion failure (TLF: cardiac death, target vessel-related myocardial infarction, TLR) compared with a prespecified performance goal based on prior-generation BMS. All major cardiac events were independently adjudicated. Dual antiplatelet therapy was required for a minimum of one month post procedure. Nine-month outcomes of the OMEGA study showed a low rate of TLF, revascularisation and stent thrombosis. The primary endpoint was met – the nine-month TLF rate was 11.5% and the upper one-sided 95% confidence bound of 14.84% was less than the prespecified performance goal of 21.2% (p<0.0001). Event rates were low, including a stent thrombosis rate of 0.6% at nine months. Through nine months, the myocardial infarction rate was 3.7% (12/326). All of the myocardial infarctions were non-Q wave myocardial infarctions, with most occurring within one day of the procedure (10/12). This supports safety and efficacy of the novel, platinum chromium OMEGA/REBEL BMS for the treatment of coronary artery disease (Figure 1).10
The REBEL(™) stent
The REBEL™ stent system is the newest advance in BMS technology. Radial strength and low recoil of stent are particularly important, as TLR rates are generally higher for BMS compared with DES. The platinum chromium alloy combined with the REBEL™ customised stent architecture allows for high radial and axial strength and the lowest stent recoil. REBEL™ is based on the OMEGA™ stent, but utilises a slightly modified stent platform with additional connectors added to the proximal end of the stent to reduce the potential for stent deformation. In bench testing, the REBEL™ stent outperforms its competitors in deliverability tests such as trackability and pushability, which are two important requirements for physicians when delivering a BMS. Platinum chromium alloy gives the best-in-class visibility during coronary stenting (if comparing with other BMS platforms). Platinum has over twice the density of iron or cobalt, resulting in enhanced visibility under fluoroscopy.11
REBEL has another useful feature: the post-dilatation overexpansion limit to 5.75mm for the large vessel stent model, resulting in optimal post-dilatation capability for big vessels.
Optimal implantation technique
Although DES have superior outcomes to BMS in terms of both restenosis and stent thrombosis, we have to agree that there is still a place for BMS in modern catheterisation laboratory. Moreover, with the optimal stent implantation technique we can achieve less TLR and stent thrombosis in both BMS and DES systems.
Colombo et al has shown that when using intravascular ultrasound, the majority of stents are not adequately deployed, despite a successful angiographic result. It was therefore hypothesised that the major cause of stent thrombosis was incomplete expansion rather than intrinsic thrombogenic properties of the device.12
In 1998, De Jaegere et al validated the safety and feasibility of intravascular ultrasound-guided stenting and its impact on the six-month restenosis rate in the Multicenter Ultrasound Stenting in Coronaries (MUSIC) study.13 They confirmed that, in selected patients, stents could safely be implanted without the use of systemic anticoagulation, when optimal stent expansion was achieved (Table 2). Of all variables predictive of stent thrombosis, the final angiographic result reflecting the degree of stent expansion has consistently been shown to be one of the most important predictors. These data corroborate previous studies in which it was found that the lumen diameter immediately after the procedure was one of the most powerful independent predictors of coronary patency at six months.
However, the optimal implantation technique and adjunctive pharmacological treatment still need to be established. The risks from aggressive stent expansion are coronary rupture or extensive vessel wall injury, which in turn may provoke a severe vessel wall response and thus restenosis. Appropriately sized balloons based on intravascular ultrasound measurements can avoid this. This may, in part, explain the low restenosis rate observed in the MUSIC study.
Conclusions
A significant proportion of patients still receive BMS. From many BMS types, the operator should use the BMS with the best performance and the lowest rate of TLR. The optimal stent implantation technique including postdilation with non-compliant balloon is compulsory. Intravascular ultrasound can help to choose right diameter stent and balloon size. As well as right lesion pretreatment, predilatation with balloons, cutting/scoring balloons, rotablator, etc, is mandatory for optimal stent implantation.
Key points
- Despite the introduction of drug-eluting stent (DES) more than ten years ago, bare metal stents (BMS) are still being used, although with varying frequency.
- The 2010 European Society of Cardiology guidelines on myocardial revascularisation recommended DES usage in almost all patients, but there are some relative clinical contraindications to the use of DES and there is a place for BMS.
- BMS are still often used in elderly patients, patients with peptic/gastric ulcers, patients with planned surgery and in those who need long-term anticoagulant therapy.
- As we already know from the MUSIC study, there are IVUS criteria of optimal stent expansion to avoid late restenosis. Using right diameter balloons and appropriate stents, we can achieve low restenosis rate. In the latest prospective, multicentre, single-arm study, Omega, nine-month outcome showed a low rate of TLF, revascularisation and stent thrombosis. Target lesion revascularisation rate was 7.4% at nine months.
- The newest BMS is the REBEL stent, which is a platinum chromium alloy stent system with lowest stent recoil, with additional stent connectors on the proximal end. REBEL platinum chromium alloy gives the best-in-class visibility during coronary angioplasty.
References
- Hoffmann R et al. Patterns and mechanisms of in-stent restenosis: a serial intravascular ultrasound study. Circulation 1996;94:1247–54.
- Silber S et al. Unrestricted randomized use of two new generation drug-eluting coronary stents: 2-year patient-related versus stent-related outcomes from the RESOLUTE All Comers trial. Lancet 2011;377:1241–7.
- Raber L et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation 2012;125:1110–21.
- Moses JW et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349:1315–23.
- Stone GW et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med 2004;350:221–31.
- Haine SE et al. Difference in clinical target lesion revascularization between a silicon carbide-coated and an uncoated thin strut bare-metal stent: The PRO-Vision Study. Can J Cardiol 2013;29:1090–6.
- European Society of Cardiology guidelines on myocardial revascularization 2010. Eur Heart J 2010;31:2501–55.
- Bangalore S. Trend in the use of drug eluting stents in the United States Insight from over 8.1 million coronary interventions. Int J Cardiol 2014;175:108–19.
- Beijk MA et al. Genous endothelial progenitor cell capturing stent vs. the Taxus Liberte stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: a randomized, single-center, pilot study. Eur Heart J 2010;31:1055–64.
- Wang JC et al. CRT-152 nine-month primary endpoint results of the Omega study: clinical outcomes after implantation of a Modern Platinum Chromium Bare Metal Stent. J Am Coll Cardiol Intv 2014;7(2_S):S1-S1. doi:10.1016/j.jcin.2013.12.007.
- Boston Scientific Internet resources. www.bostonscientific.com/interventional-cardiology-eu/index.html? (accessed date 18 July 2014).
- Colombo A et al. Intracoronary stenting without anticoagulation accomplished with intravascular ultrasound guidance. Circulation 1995;91:1676–88.
- de Jaegere P et al. Intravascular ultrasound-guided optimized stent deployment. Eur Heart J 1998;19:1214–23.