
While a significant proportion of patients with myocardial infarction are prescribed clopidogrel, common genetic variants mean that some people – and more from certain ancestry groups – may not see its preventative benefit.
Here, Dr Emma Magavern, clinical research fellow for the Centre of Clinical Pharmacology and Precision Medicine at William Harvey Research Institute, Queen Mary University of London, UK, discusses her most recent findings and the value of clinicians adopting pre-emptive pharmacogenomic testing.
As clinicians, we know that there is inter-individual variability in medication response. Different people respond differently to the same medication; some take medication and do not experience the intended benefit, and some people unfortunately experience adverse drug reactions (ADRs). Some of this variability in response is due to genetics.
We already use other information known to impact on risk of ADRs from a medication in our clinical practice. Examples include checking liver and renal function before prescribing medicines.
By using information on very common genetic markers to inform prescribing, we can optimise the benefits of medications and minimise the risk of side effects. This area, known as pharmacogenomics, has however been slow to enter mainstream clinical practice.
The recently published PREPARE trial showed that a pre-emptive pharmacogenomic testing approach, where the genotype informs prescribing, can decrease ADRs by one-third in a multi-centre prospective clinical trial setting.1
ADRs pose significant problems for patients and take a large toll on healthcare systems. Studies estimate ADRs are responsible for 6.5% of hospital admissions in the UK and cost more than £2bn a year to the NHS.2,3 So there is clearly much room for improvement.
Clopidogrel and genetic resistance
People from certain ancestry groups may have a higher risk of experiencing a side effect or not benefiting from medication due to common genetic variants. An example is illustrated by the use of clopidogrel after a myocardial infarction.
Clopidogrel is a commonly prescribed P2Y12 inhibitor used for secondary prevention after an initial ischaemic event.4 It is a prodrug and must therefore be activated in the body, via metabolism by cytochrome P450 2C19 (CYP2C19) in the liver, to achieve potency. Studies have shown that 60–70% of people who suffer from a myocardial infarction are prescribed clopidogrel.5
The CYP2C19 enzyme is encoded by the CYP2C19 gene, which is highly polymorphic.6 People with CYP2C19 loss of function (LOF) variants have higher on-treatment platelet reactivity with clopidogrel and are at a greater risk of secondary ischaemic events.7
LOF variants in CYP2C19 are common – present in approximately one in three people of European ancestry.7 Therefore, genetic variants resulting in individuals not being able to activate clopidogrel are common.
However, these variants are even more common in certain ancestral groups. Genetic changes that lead to poor activation of clopidogrel are most common in Asian and Oceanic ancestry groups.8
So, these ancestry patients have a higher risk of clopidogrel being ineffective than European ancestry participants, in whom antiplatelets were predominantly trialled, and therefore might benefit most from the use of genetics to help determine clopidogrel safety and efficacy (see Table 1 below).
Few studies have linked genetic data from under-represented populations with medication exposure and health outcome data. The only study with a substantial Asian population was focused on stroke rather than myocardial ischaemia and undertaken in east Asia.
Table 1. Studies supporting clopidogrel licensure as listed in the European Medicines Agency summary of product characteristics9–16
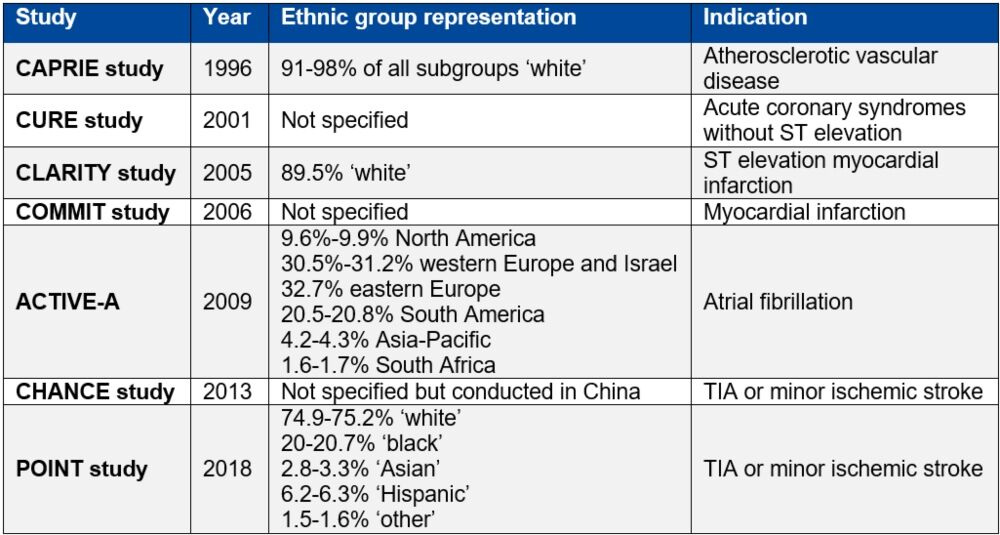
Table reproduced from Magavern E et al. JACC Adv 2023;2(7):100573. Copyright © 2023, The Authors. Open Access Article under the CC BY License.
Analysing the Genes & Health study
The British-Bangladeshi and British-Pakistani populations in the UK are known to experience high rates of cardiovascular disease. The Genes & Health study recruited more than 50,000 people from this population and linked genotype data with national electronic health records data and primary care prescription records.17
In an analysis of 44,396 participants, we found that 57% of people in this group had a CYP2C19 LOF variant.18 Of these, 13% had two copies of an LOF variant – one from each parent (Figure 1).
Figure 1. Prevalence of CYP2C19 LOF alleles in the Genes & Health study’s South-Asian ancestry population
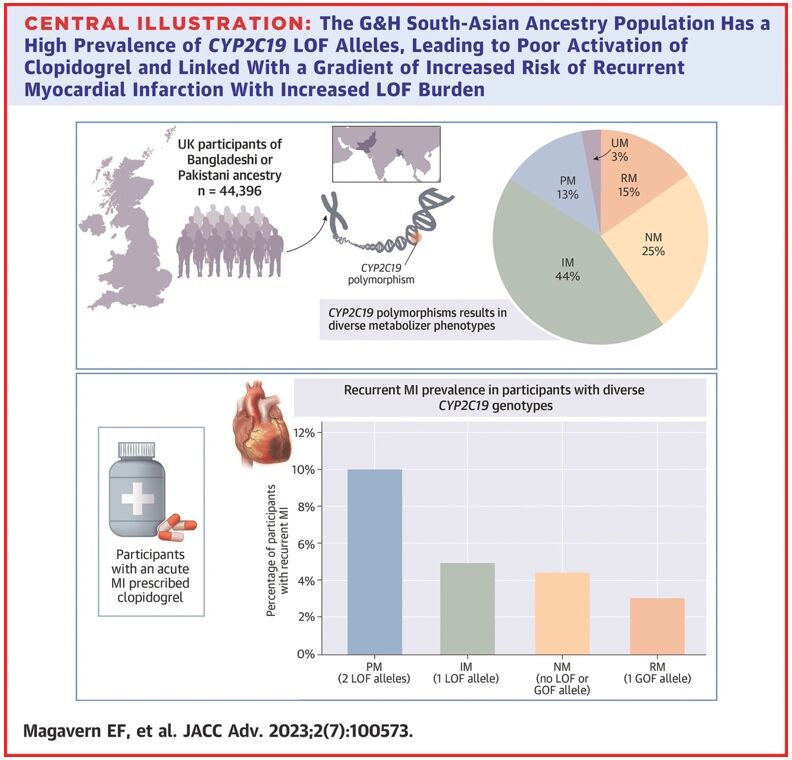
Reproduced from Magavern E et al. JACC Adv 2023;2(7):100573. Copyright © 2023, The Authors. Open Access Article under the CC BY License.
We identified people in this group who had experienced a myocardial infarction and were prescribed clopidogrel by their GP (697 participants). Those with a recurrent myocardial infarction were almost four-times more likely to have two LOF CYP2C19 alleles as compared with none. A total of 69% of participants who had a myocardial infarction were prescribed clopidogrel.18
This study showed how common genetic resistance to clopidogrel is in this population. It also demonstrated the widespread use of clopidogrel and linked genetic resistance with a higher risk of recurrent myocardial infarction in this population for the first time, using real-world data.
While a precision prescribing approach using CYP2C19 testing would benefit patients from all ancestries, it would disproportionately benefit the South-Asian ancestry population due to the higher prevalence of both coronary artery disease and genetic resistance to clopidogrel in this population.
Implementing genetic testing
Genetic testing may soon be available within the NHS to determine if stroke patients would benefit from clopidogrel.19 This means that some patients might already know their CYP2C19 genotype when they present with a secondary ischaemic event.
This information could subsequently be used to administer the most effective medicine and reduce their risk of further ischaemic events.
For pharmacogenetic testing to benefit patients who have had a myocardial infarction, several things must occur:
- Regulatory bodies must recommend genetic tests and make them available
- Healthcare practitioners must be able to access the results in a timely fashion and have clinical decision-support tools to guide the use of this information
- Healthcare pathways must facilitate access to, and storage of, this information.
There should also be discussion with the public around this use of genetic testing, to raise awareness and design clinical pathways fit for purpose. These dialogues must include historically under-represented ancestry groups and build trust.
Conclusion
The goal is to make genetic testing possible in routine clinical care to help optimise antiplatelet choice after an ischaemic event and to ensure better outcomes for all, with a particular focus on improving health equality for the British South-Asian ancestry population.
Author
Emma Magavern MD MSc MRCP
Clinical Research Fellow, Centre of Clinical Pharmacology and Precision Medicine, William Harvey Research Institute, Queen Mary University of London, UK
References
- Swen JJ et al. A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet 2023;401:347–56
- Pirmohamed M et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18,820 patients. BMJ 2004;329:15–19
- Osanlou R et al. Adverse drug reactions, multimorbidity and polypharmacy: a prospective analysis of 1 month of medical admissions. BMJ Open 2022;12 e055551
- National Institute for Health and Care Excellence. BNF: Clopidogrel – Medicinal forms. 2022 (accessed March 2024)
- Turgeon RD et al. Association of Ticagrelor vs Clopidogrel With Major Adverse Coronary Events in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. JAMA Intern Med 2020;180:420
- Scott SA et al. PharmGKB summary. Pharmacogenet Genomics 2012;22:159–65
- Lee CR et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2C19 Genotype and Clopidogrel Therapy: 2022 Update. Clin Pharmacol Ther 2022; doi:10.1002/cpt.2526
- PharmGKB and CPIC. Gene-specific Information Tables for CYP2C19. 2022 (accessed March 2024)
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348:1329–39
- Yusuf S et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502
- Sabatine MS et al. Addition of Clopidogrel to Aspirin and Fibrinolytic Therapy for Myocardial Infarction with ST-Segment Elevation. N Engl J Med 2005;352:1179–89
- Chen ZM et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1607–21
- ACTIVE Investigators et al. Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation. N Engl J Med 2009;360:2066–78
- Wang Y et al. Clopidogrel with Aspirin in Acute Minor Stroke or Transient Ischemic Attack. N Engl J Med 2013;369:11–19
- Johnston SC et al. Clopidogrel and Aspirin in Acute Ischemic Stroke and High-Risk TIA. N Engl J Med 2018;379:215–25
- Electronic Medicines Compendium. Clopidogrel
- Genes & Health. (accessed March 2024)
- Magavern E et al. CYP2C19 genotype prevalence and association with recurrent myocardial infarction in British-South Asians treated with clopidogrel. JACC Adv 2023;2(7):100573
- National Institute for Health and Care Excellence. News. Testing could help prevent further strokes in people with gene variant. 2023 (accessed March 2024).
This article is part of our Clinical Excellence series, which offers valuable first-hand insights into how experts from renowned Centres of Excellence are pursuing innovative approaches to optimise patient care across the UK and Europe.










