DePuy Synthes Mitek Sports Medicine*, a leader in orthopaedics sports medicine, has announced it has added the RIGIDFIX® Curve Cross Pin System, the first of its kind, designed specifically for use with the anteromedial (AM) portal approach to enable a more anatomic ACL (anterior cruciate ligament) reconstruction.
The company also announced the launch of the RIGIDLOOP™ Cortical Fixation System, which when used with the INTRAFIX® ACL Tibial Fastener System, provides surgeons with a total procedural solution for anatomic soft tissue ACL reconstructions.
Both systems were unveiled at the 15th European Federation of National Associations of Orthopaedics and Traumatology (EFORT) congress, a combined programme in partnership with the British Orthopaedic Association, held in London, UK. In addition, the company announced that, along with the 23mm screw which was released earlier this year, two new sizes of MILAGRO® ADVANCE Interference Screws (30mm and 35mm) were added to Mitek Sports Medicine’s growing portfolio of knee solutions for soft tissue and bone-patellar tendon-bone reconstruction.
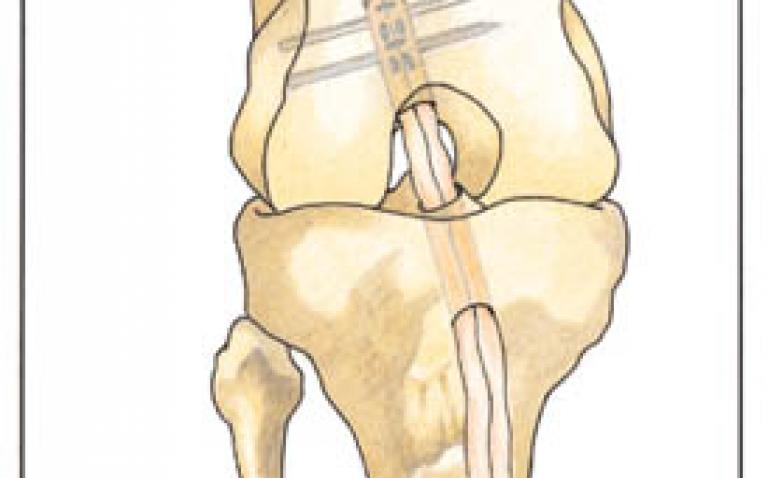
RIGIDFIX Curve Cross Pin System
Using the AM portal approach, the RIGIDFIX Curve System enables precise placement of two cross pins in the femoral tunnel. The cross pins provide strong fixation and the close to aperture fixation minimises intratunnel graft micromotion, small movements of the graft inside the femoral tunnel, that may cause tunnel widening.(1) The two cross pins create a compression fit that further enhances 360-degree graft to bone contact. The design of RIGIDFIX Curve is based on the original RIGIDFIX® Cross Pin System, which has been used for ACL reconstruction through a transtibial approach for more than 10 years.
“Many surgeons are nowadays moving from transtibial drilling to an anteromedial approach, as it may provide a greater rotational stability of the knee due to the more anatomic placement of the graft,” said Peter Reynaert, Consultant Orthopaedic Surgeon, Heilig Hart Hospital, Leuven, Belgium. “For this approach, RIGIDFIX Curve provides a unique fixation with well known biomechanical advantages and has features that enhance precision and ease-of-use.”
RIGIDLOOP Cortical Fixation System
The RIGIDLOOP system offers a strong, easy-to-use solution for soft tissue ACL reconstructions. The implant consists of a titanium button that comes pre-loaded with a braided suture loop and offers excellent pull out strength.(2) The new device is available in sizes 15mm-60mm in 5mm increments, as well as an Extra-Large (XL) button for femoral tunnels greater than 6mm in diameter. Designed with simplicity in mind, the system includes an innovative variable depth gauge that calculates total transosseous length, amount of graft in tunnel, socket reaming depth and implant size. When used in combination with Mitek Sports Medicine’s INTRAFIX ACL Tibial Fastener System, the market’s leading tibial fixation device, the RIGIDLOOP System provides a complete anatomic soft tissue ACL reconstruction which consists of the strongest cortical device(2) (compared to Endobutton) and strongest, clinically proven tibial device.(3)
Also at EFORT, Mitek Sports Medicine announced that the MILAGRO ADVANCE Interference Screw is now available in larger sizes–30mm and 35mm. MILAGRO ADVANCE Interference Screw is a biocomposite screw designed to offer easier and faster insertion when compared to traditional interference screws.(4)
“Our portfolio of orthopaedic sports medicine solutions continues to grow as we are introducing new innovative products to meet the clinical needs of patients and surgeons,” said Rocco De Bernardis, Marketing Director, Mitek Sports Medicine. “Our new products for ACL reconstruction were designed to accommodate surgeons’ different procedural preferences, but likewise the common need for strong fixation, procedural simplicity, reproducibility and a more anatomic reconstruction. Among them, RIGIDFIX Curve is not only a new product it is also a “procedural innovation”, introducing new thinking and ideas on how ACL reconstructions can be performed.”
About Mitek Sports Medicine
At Mitek Sports Medicine, we are passionate about getting patients back to their passion. As a global leader in orthopaedic sports medicine, we develop minimally invasive devices and non surgical products used in the treatment of joint injuries related to sports and physical activity, as well as degenerative tissue conditions. Mitek Sports Medicine is part of the DePuy Synthes Companies of Johnson & Johnson, the largest provider of orthopaedic and neurological solutions in the world. For more information, visit www.depuysynthes.com.
*DePuy Synthes Mitek Sports Medicine is a division of DePuy International Ltd. The third party trademarks used herein are the trademarks of their respective owners.
References
- Joshua A. Baumfeld, etal. Tunnel widening following anterior cruciate ligament reconstruction using hamstring autograft: a comparisonbetween double cross-pin and suspensory graft fixation. Knee Surg Sports Traumatol Arthrosc (2008) 16:1108–1113
- Internal test data, ref. DHF-102035-MEMO 14 &16 (compared to Endobutton)
- Kousa P, etal. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part II: tibial site. Am J Sports Med 2003
- Internal test data, ref. DHF-101914-DFTR and DHF-101914-DFTR2