Bimekizumab is the first agent that inhibits the two cytokines IL-17A and 1L-17F which are both involved in the development of psoriasis.
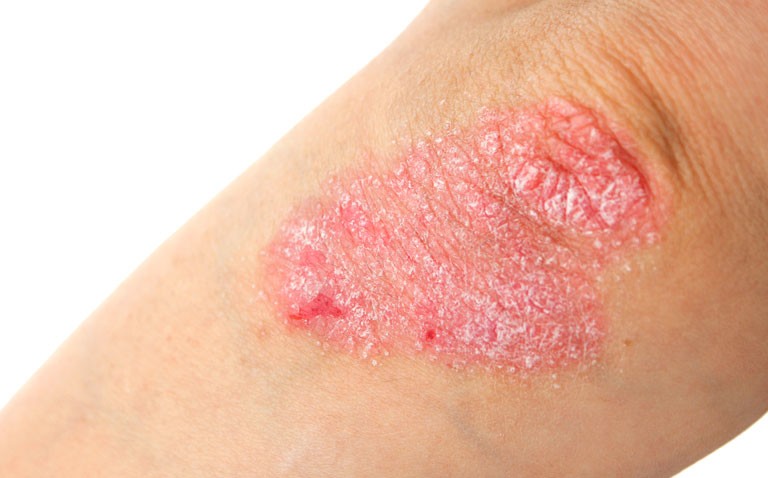
Psoriasis is best described as a complex, chronic, multifactorial and inflammatory disease characterised by increased proliferation of keratinocyte cells in the skin giving rise to silvery/white plaques. The disease typically presents on extensor surfaces such as the elbows, knees and lower back and there if often involvement of the scalp. The incidence of psoriasis appears to vary across the world, with a recent study noting a wide variation. For instance, the incidence was 30.3 per 100,000 person years in Taiwan but 321 per 100,000 person years in Italy.
Among those with psoriasis, one study of over 9,000 patients, found that just over half (51.8%) had mild disease, with 35.8% and 12.4% having moderate and severe disease respectively. Patients with mild to moderate disease are normally managed with topical therapies, whereas those with moderate to severe disease are increasing treated with biological agents, in particular, monoclonal antibodies. Although the precise cause of psoriasis remains to be determined, several interleukins appear to be important disease drivers, especially the interleukin-17 (IL-17) pathway which includes six similar agents, IL-17A – IL-17F.
Research suggests that two members of the IL-17 family, IL-17A and IL-17F are implicated in autoimmunity and IL17F appears to regulate pro-inflammatory gene expression and which requires the IL-17A receptor, suggesting that both are involved in the pathology of psoriasis. Bimekizumab is the first monoclonal antibody which selectively inhibits both IL-17A and IL-17F and is therefore a potentially important advancement in the management of patients with moderate to severe psoriasis.
Clinical efficacy
The EU approval of bimekizumab was supported by the results of three phase 3 clinical trials, all undertaken in patients with moderate to severe psoriasis. The first, BE READY, randomised 435 patients (4:1) to bimekizumab 320 mg every 4 weeks or placebo. The co-primary endpoint was a PASI90 (i.e., 90% improvement in disease severity compared to baseline) and the proportion of patients achieving a score of 0 (i.e., clear skin) or 1 (almost clear), based on a 5-point investigator global assessment (IGA) score after 16 weeks of treatment.
The results showed that a staggering 91% of those assigned to bimekizumab achieved a PASI90 compared to only 1% in the placebo group. In the BE VIVID trial, 567 patients were randomised to bimekizumab (at the same dosage as the BE READY trial) or this time, ustekinumab 45 or 90 mg every 12 weeks and which is an active comparator or placebo. Once again at week 16 there was a high response in the bimekizumab group with 85% achieving a PASI90 compared to 50% in the ustekinumab. The third trial, BE SURE, enrolled 478 patients who were randomised to either bimekizumab (same dosage as in the other trials) or adalimumab (another active comparator) at a dose of 40 mg every 2 weeks. At week 16, a PASI90 was achieved 85.3% of those using bimekizumab and 57.2% of those given adalimumab.
The most common adverse effects from bimekizumab are upper respiratory tract infections (14.5%) and patients are advised to seek medical advice if they display symptoms suggestive of an infection.
The EU approval applies to all 27 member states as well as Iceland, Liechtenstein and Norway at a dose of 320 mg administered by subcutaneous injections every 4 weeks for week 16 and every 8 weeks thereafter. The drug is currently under review by the US Food and Drug administration.