Patient dosimetry audit is a legal requirement in the UK under the Ionising Radiation (Medical Exposure) Regulations (IRMER)1 in order that that diagnostic reference levels (DRLs) can be established and used for X-ray imaging examinations. The principles are also applicable internationally as IRMER is itself based on European legislation and recommendations of the International Commission on Radiological Protection (ICRP). DRLs represent radiation dose levels that would be considered typical for a standard patient. They are a means of monitoring patient doses and are a guide to ‘good and normal practice’,2 allowing consistently high doses to be identified and investigated. Patient dosimetry audit is the analysis of data relating to patient doses to calculate average dose indicator values (usually dose length product (DLP) for CT), check adherence to existing DRLs and set new ones where there is enough data.
Patient dosimetry audit is a well established practice in diagnostic radiology. In the UK, National DRLs (NDRLs) are set by Public Health England (PHE)3–5 and guidance on establishing Local DRLS (LDRLs) has been in place for around 15 years.2 The use of electronic systems such as computed radiological information systems (CRIS) has had a profound impact on the scale and efficiency of this process.6,7 Our local system at RRPPS (the Radiation Protection Services of University Hospitals Birmingham NHS Foundation Trust, UK) is based on CRIS downloads analysed using an in-house python software to give average dose indicators by examination and room.
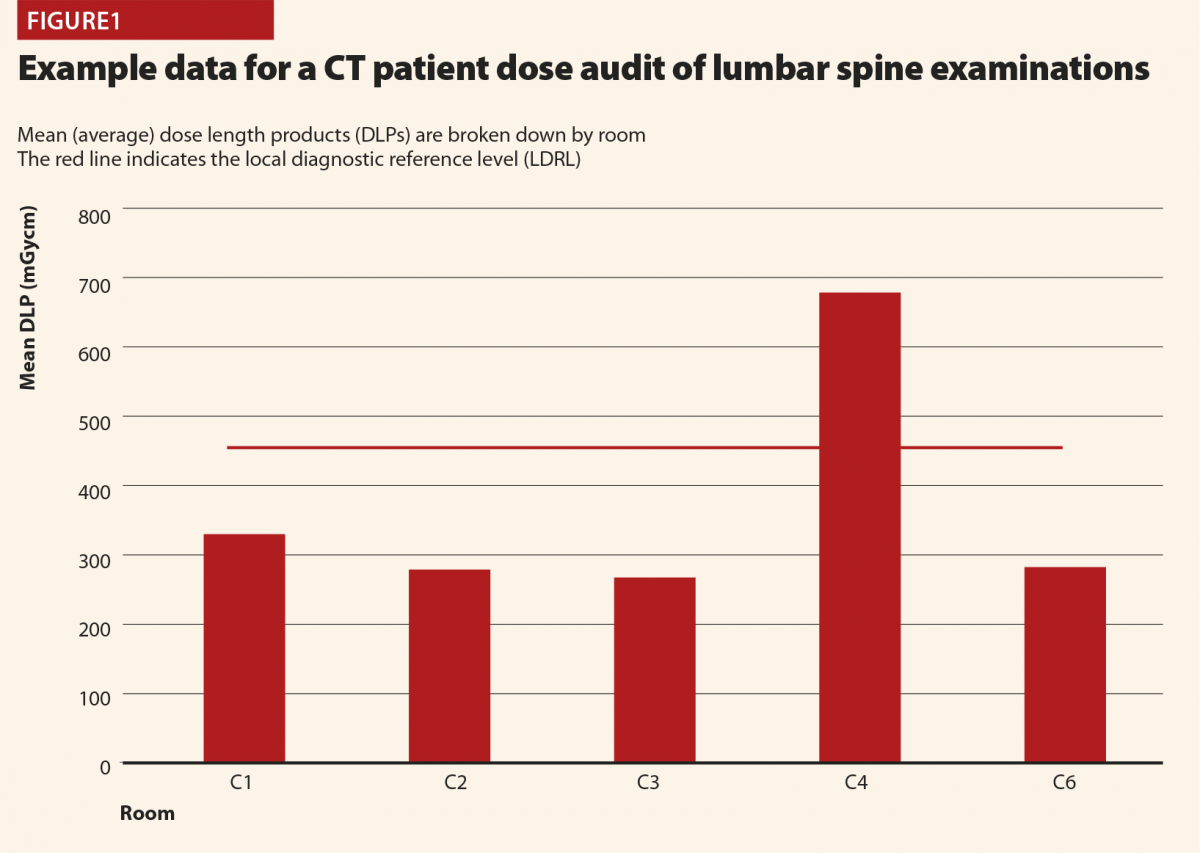
Figure 1 demonstrates how LDRLs are used and established, and gives example data from an audit of CT lumbar spine examinations at a large UK hospital. Mean (average) doses for individual rooms can be compared against an LDRL, which is set as the mean of individual room means. In this case there is clearly a problem with scanner 4, on which the LDRL is consistently exceeded. Having identified that an issue exists via patient dosimetry audit, investigations and corrective action can be implemented. In this case the investigation revealed that whilst all of the scanners were equipped with tube-current modulation, scanner 4 had been set up with a much high reference tube current than the others. This was rectified to harmonise the protocols across all scanners. Additional cases have identified scanners with subtly different imaging protocols and tube-current modulation not being switched on! When there can easily be dozens or more protocols set up on CT scanners, it is usually impractical to check each one line-by-line and compare across every scanner, but simple setup errors will go on to influence doses for many patients. Using patient dosimetry audit to identify such issues and harmonise protocols and practices helps identify and avoid the situation of patients receiving significantly different radiation doses depending on which scanner they happen to find themselves on. In some cases, it might be justified for a particular scanner to have different protocols, and therefore give different doses to others, perhaps because it is used for different clinical conditions (for example, trauma scans). But where there is no such reason, harmonisation of protocols is a simple means of dose optimisation.
CT is also used as standard outside of diagnostic radiology such as in nuclear medicine (with SPECT/CT and PET/CT being routine) and radiotherapy (for treatment planning scans and on-board imaging for verifying patient positioning prior to treatment). Only in the past few years has there been progress in establishing patient dosimetry audit in these areas.8–12 In encouraging recent progress, the Institute of Physics and Engineering in Medicine (IPEM) has established working parties that undertook national audit and published data for nuclear medicine CT13 and radiotherapy planning CT,14 the results of which were subsequently adopted as UK NDRLs.5 This gives very useful data for local results to be compared against. The rest of this discussion will be on the efforts based at RRPPS to establish local systems for patient dosimetry audit in these areas, including some of the challenges we encountered and the solutions developed. In the future, it is hoped similar work will be carried out for radiotherapy on-board imaging as well.
Nuclear medicine CT
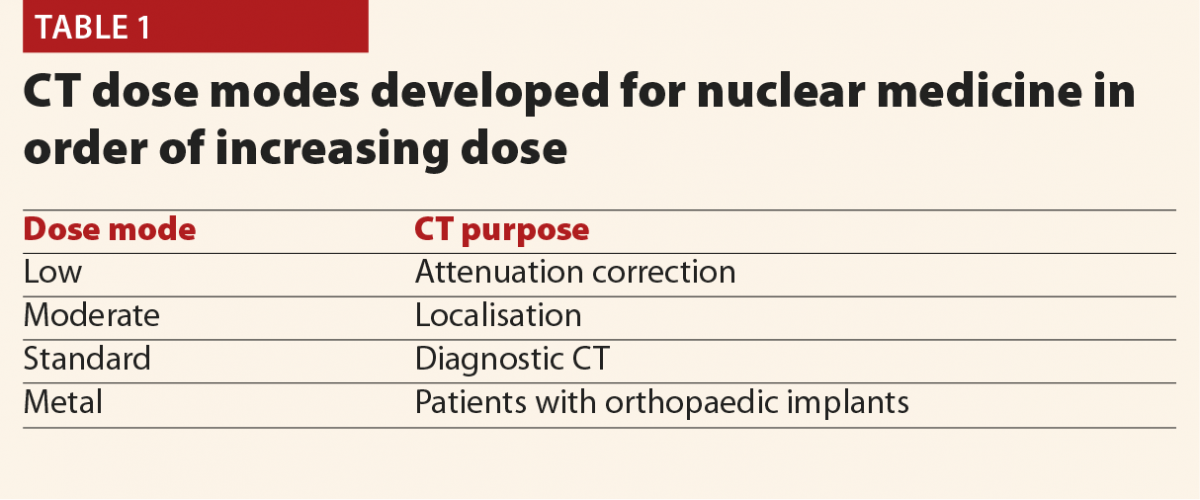
The full results of the initial patient dose audit were published in the British Journal of Radiology15 based on the proposed NDRLs which were available at the time.16 Data were collected for adult patients on two SPECT/CT Scanners (Siemens Symbia T & T16) over November 2014–July 2016 and for a PET/CT scanner (Siemens Biograph mCT Flow) over April–August 2016. Initially, we based the audit on the nuclear medicine examination type (bone scan, parathyroid, etc) as this matches how the NDRLs were set out. However, it soon became apparent that nuclear medicine CT has some complications not found in conventional CT, and so there was more to consider. In discussions between diagnostic radiology and nuclear medicine physicists, two additional considerations were identified; dose mode and body region. Dose mode accounts for the fact that different CT scans had different purposes; for some the CT data was simply used for attenuation correction and so a lower CT dose is needed. Others are full diagnostic quality CT scans, needing higher doses. Table 1 summarises the four dose modes in use in our hospital’s nuclear medicine department. Body region arises due to the use of SPECT-guided CT, in which only the part of the body of interest on the SPECT scan undergoes CT. This is to be encouraged, as it means that the patient’s exposure to X-rays is limited, but does also mean that CT scan lengths vary according to what is scanned and we begin to see descriptions which resemble diagnostic radiology CT; head, t-spine, abdomen and so on as well as the dose mode and examination type. So we must break down the data in order to audit and compare like-for-like. Unfortunately not all of these additional data are captured as standard in CRIS downloads, our standard source of data for patient dosimetry audit. CRIS records captured the nuclear medicine examination type and DLP, so paper records completed after each scan had to be modified to capture dose mode, body region and the scanner used.
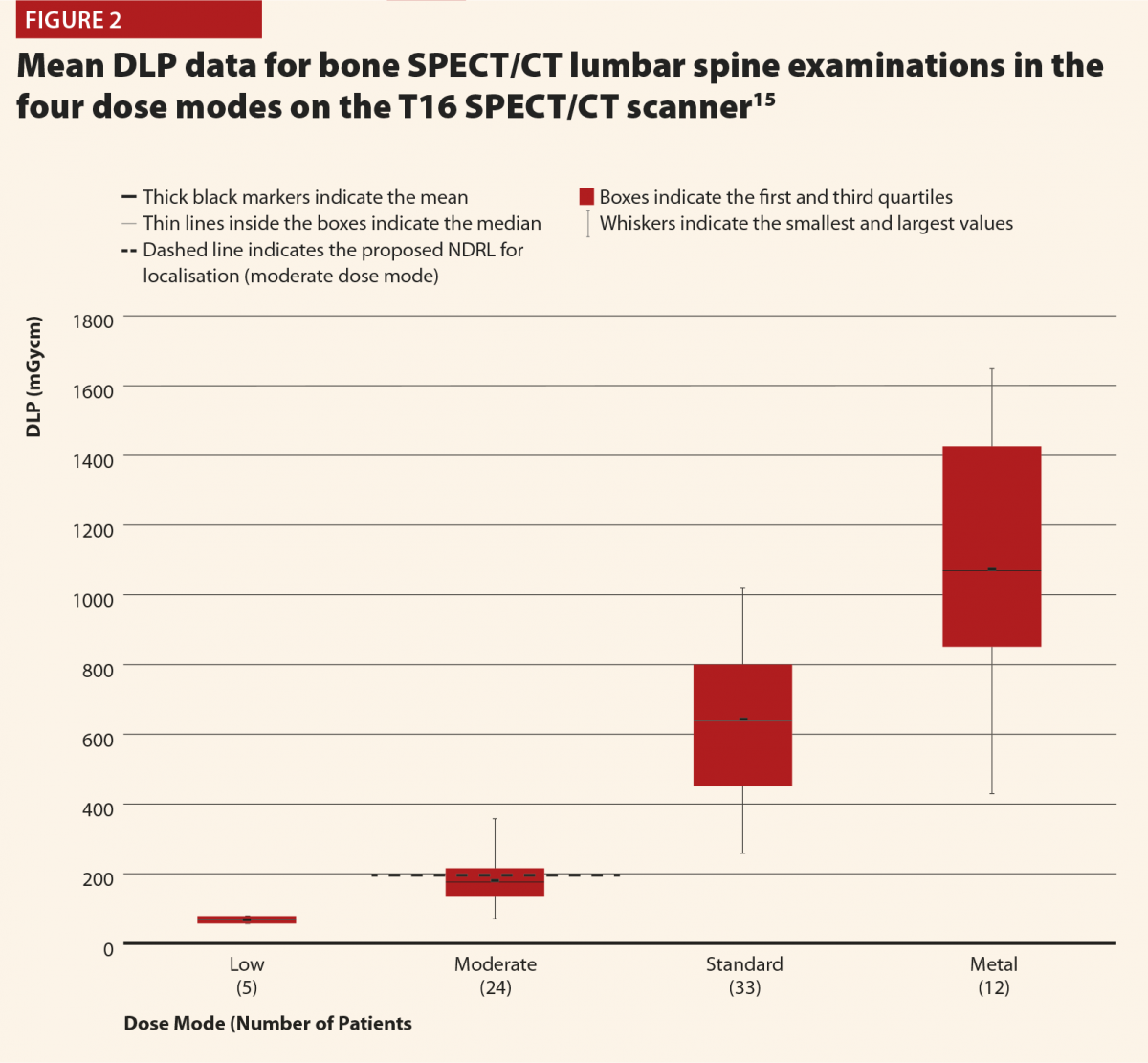
Figures 2 and 3 give selected results from this initial audit,15 which highlight some important points. Figure 2 clearly shows that the different dose modes result in significantly different doses, and so they must be considered separately in order to complete a reliable audit. This also highlights that the NDRLs are currently somewhat limited in that they do not fully consider the range of scan purposes, although of course it must be acknowledged that there are limited data currently available and this will hopefully improve in time.
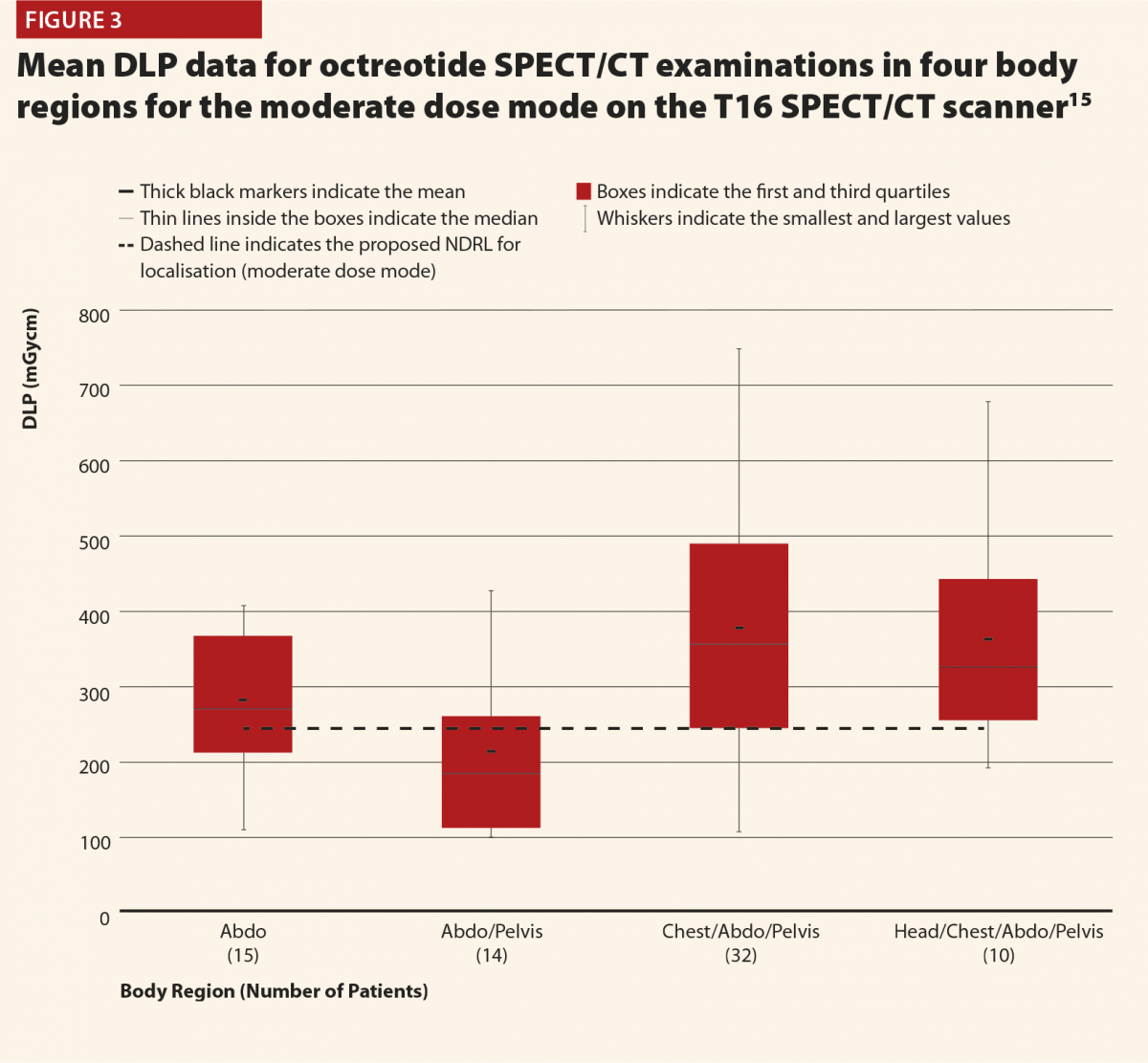
While Figure 3 should be treated with some caution as the numbers of patients are quite low, there is still some useful insight to be gained. There does appear to be a trend that the CT scans covering more than the chest, abdomen and pelvis or more give notably higher doses (and consistently exceed the proposed NDRL). This is to be expected, as longer scans of course give larger DLPs. This raises the question of is there a genuine concern about this exceeded NDRL? This NDRL is set for octreotide examinations, and would therefore represent a collation and average across all body regions scanned. It seems unfair therefore to apply it to longer scans, as they are achieving their clinical aim but will inherently struggle to work within DRLs set based on shorter scans. Further study of the NDRL publication13 reveals that the octreotide data used in this national audit are based primarily on CT scans of the abdomen, meaning that it may not be appropriate to apply the NDRL for longer scans. This highlights the need to consider body regions separately when carrying out the audit.
Overall there are a number of challenges in patient dosimetry audit for nuclear medicine CT. Far fewer nuclear medicine CT scans are carried out compared with radiology CT, and the need to subdivide the data into dose modes and body regions can make it difficult to accumulate sufficient quantity of data for a reliable audit. A solution is to accumulate data over longer periods of time; perhaps two years instead of annually. CRIS did not capture all of the information needed, resulting in a reliance on paper records being transcribed for analysis electronically. This is a very time-consuming process and can result in possible transcription errors. Another potential problem with paper records is inaccurate or inconsistent naming conventions, especially for body regions. For instance, some technologists might write ‘lumbar spine’ and others ‘L-spine’, meaning that any automated analysis will treat these as separate. A significant amount of manual interrogation of data is therefore required to ensure data is recorded correctly and consistently, a very time consuming and inefficient process.
Following on from the initial audit, we have been able to implement improved local systems to facilitate routine audit in a more efficient manner. These include collecting SPECT/CT over a two-year audit period to ensure enough data are included to be confident in it. Additional CRIS entries have been found to record dose mode, and a free text box is used to record body region, removing the need for paper records. Principal technologists also developed guidance on naming conventions to help achieve consistency and reduce the burden of manually correcting entries retrospectively. Finally, a monthly CRIS download is performed and reviewed by a nuclear medicine assistant practitioner to check adherence to naming conventions, confirm the scanner used and body region, and check any ambiguous or suspicious data. These monthly checks are essentially a tidy and quality check of the data, with monthly numbers being found to be more manageable. Therefore the system is not fully automated, but much less labour intensive than previously and with practices improving over time, routine audit can be made sustainable in the long term.
In summary, it is certainly possible to develop systems for patient dosimetry audit in nuclear medicine CT, but auditors must be aware of the additional complications that might not be familiar to those primarily working in diagnostic radiology. Careful thought is needed on how these complications will be managed in an efficient way to allow for ongoing audit.
Radiotherapy planning CT
For radiotherapy planning CT, data were analysed for adult patients on two Philips Brilliance Big Bore CT scanners over the 2017 calendar year. The task was found to be very similar to that for diagnostic radiology CT, in that there were no separate dose modes and the protocols were standardised in terms of the body region scanned. Once the data were acquired, it was therefore simple to analyse using established methods, but there were challenges with the practicalities of acquiring the data. Radiotherapy systems are not interfaced with CRIS, as the scans are not taken to be reported on but to facilitate treatment. Instead, radiotherapy systems include treatment planning and record and verify systems. These systems do not appear to be designed with patient dosimetry audit in mind. It is a simple matter to look up the records of individual patients, but these systems are not set up for outputting large-scale patient dose information in the format facilitating audit (such as a spreadsheet). So a solution is needed to avoid manually combing individual patient records one at a time for data, an extremely inefficient proposal.
An initial audit showed that radiographers had been keeping paper records of all CT scan dose information (largely for historical reasons) and so these data were transcribed for analysis. These records did not specify the protocol used but the examination purpose and so there could be issues with naming conventions, with different description actually referring to the same type of scan. Knowing the protocol would allow such cases to be identified and merged for more reliable audit. To help resolve this, a simple in-house software was developed to search the data and extract the protocol information based on keywords in examination information. This allowed protocols to be matched against the manual descriptions and in discussion with radiographers, datasets can be merged where similar protocols are used. The hope in future is that the in-house software will be usable more widely to allow the need for manual transcription and interrogation of the data to be reduced and the data simply extracted.
So as with nuclear medicine CT, we have been able to implement a system for local audit of radiotherapy planning CT (and incidentally demonstrate compliance with NDRLs was being achieved), although with some room for further improvement to automate the process. The challenges were much more practical than analytical in this case, meaning that most of the work involved in setting up local audit is in finding efficient ways to reliably collect data on a large scale and so this may have to be carefully considered when implementing audit elsewhere.
References
1 Ionising Radiation (Medical Exposure) Regulations 2017. Statutory Instruments 2017, No. 1322. London, HMSO.
2 IPEM. Guidance on the Establishment and Use of Diagnostic Reference Levels for Medical X-ray Examinations. Institute of Physics and Engineering in Medicine. Report Number 88, 2004.
3 Hart D, Hillier MC, Shrimpton, PC. Doses to patients from radiographic and fuoroscopic X-ray imaging procedures in the UK – 2010 review. Health Protection Agency. Report Number: HPA-CRCE-034, 2012.
4 Shrimpton PC et al. Doses from computed tomography (CT) examinations in the UK – 2011 review. Public Health England. Report Number: PHE-CRCE-013, 2014.
5 Public Health England, 2016. Diagnostic Radiology: National Diagnostic Reference levels (NDRLs). www.gov.uk/government/publications/diagnostic-radiology-national-diagnos… (published 15 November 2018) (accessed August 2019).
6 Charnock P, Moores BM, Wilde R. Establishing local and regional DRLs by means of electronic radiographical X-ray examination records. Radiat Prot Dosimetry 2013;157:62–72.
7 Charnock P et al. Establishment of a comprehensive set of regional DRLs for CT by means of electronic X-ray examination records. Radiat Prot Dosimetry 2015;163:509–20.
8 Etard C et al. National survey of patient doses from whole-body FDG PET-CT examinations in France in 2011. Radiat Prot Dosimetry 2012;152:334–8.
9 Jallow N et al. Diagnostic reference levels of CT radiation dose in whole-body PET/CT. J Nucl Med 2016;57:238–41.
10 Alkhybari EM et al. Determining and updating PET/CT and SPECT/CT diagnostic reference levels: A systematic review. Radiat Prot Dosimetry 2018;182:532–45.
11 Dennis JL, Gemmell A, Nicol AJ. Optimization of the CT component of SPECT-CT and establishment of local CT diagnostic reference levels for clinical practice. Nucl Med Commun 2018;39:493–9.
12 Connor SO, Mc Ardle O, Mullaney L. Establishment of national diagnostic reference levels for breast cancer CT protocols in radiation therapy. Br J Radiol 2016;89:20160428.
13 Iball GR et al. A national survey of computed tomography doses in hybrid PET-CT and SPECT-CT examinations in the UK. Nucl Med Commun 2017;38(6):459–70.
14 Wood TJ et al. IPEM topical report: the first UK survey of dose indices from radiotherapy treatment planning computed tomography scans for adult patients. Phys Med Biol 2018;63:185008.
15 Gardner M et al. Patient dosimetry audit for establishing local diagnostic reference levels for nuclear medicine CT. Br J Radiol 2017;90:20160850.
16 Bebbington N et al. UK national reference doses for CT scans performed in hybrid imaging studies. J Nucl Med 2016;57(no. supplement 2):594.