The novel approach of precision medicine is also reaching the field of mechanical ventilation, with the ultimate aim of customising ventilation and providing patients with the highest quality support. Big steps have been made by manufacturers in the last years, with some new technologies already available for clinical use and which help optimise patient–ventilator interaction.1 However, the greatest effort still comes from clinicians themselves, who should examine the ventilator waveforms in order to detect mismatches between patient and ventilator inspiratory and expiratory times (these phenomena are termed asynchronies). They are observed frequently in ventilated patients and represent a failure in providing them with optimal assistance. Asynchronies have negative clinical consequences such as prolonged mechanical ventilation, difficult weaning, reduced comfort for the patient, increased risk of diaphragmatic damage and potentially increased morbidity and mortality.2–6 Asynchronies can be detected by looking at the ventilator waveforms at the patient’s bedside;7,8 a good knowledge of the phenomenon is therefore essential for diagnosis and correction.
Classification of asynchronies
There are a few different classifications of patient-ventilator asynchronies, each of them considering a different aspect of the phenomenon.9,10
Phase
Asynchronies can be classified as inspiratory or expiratory, depending on the neural respiratory phase that is affected. Inspiratory asynchronies are delayed triggering, ineffective effort and autotriggering, whilst expiratory asynchronies are late and early cycling and double triggering.
Relevance
Asynchronies can be classified in major or minor, depending on the type of assistance provided by the ventilator: if there is no correspondence at all between patient’s request and ventilator assistance (that is, the patient starts a breath but the ventilator does not provide any support), the asynchrony is ‘major’, whereas if the ventilator supports the patient in response to his/her request, but the assistance is not appropriate (delayed or not sufficient), the asynchrony is ‘minor’. Mojoli et al pointed out that minor asynchronies might have a greater impact than major ones in ventilated ICU patients.11
Aetiology
Some asynchronies are typically associated with a low patient respiratory drive and/or a too high ventilator assistance (ineffective efforts, delayed cycling, autotriggering, reverse triggering); others are associated with high respiratory drive and low ventilator support, such as early cycling and double triggering.12
Clinical relevance
The first aspect to consider is the prevalence of asynchronies: they are very common during ventilation, not only in assisted modes but also in controlled modes. In 1997, Chao et al13 observed 200 patients during the weaning from mechanical ventilation and found that 10% of them had ineffective efforts; this phenomenon was associated with prolonged and difficult weaning. This was the first large study focusing on patient–ventilator asynchronies. Following on from this, there was an increasing interest on the subject; other studies confirmed the high prevalence of asynchronies in ICU patients, clarifying their clinical impact as well. Asynchronies started to be considered not only as a cause of discomfort for patients,14 but also as a cause of prolonged mechanical ventilation,6,15 muscle injury, higher sedation requirements,16 and eventually increased mortality.4 Moreover, asynchronies could be involved in long-term neuropsychological effects in patients with respiratory distress.17,18
Clinicians applied different monitoring tools to detect asynchronies (oesophageal pressure, diaphragm electrical activity), and manufacturers produced new modes of ventilation aiming to better fit patients’ requirements (Table 1).
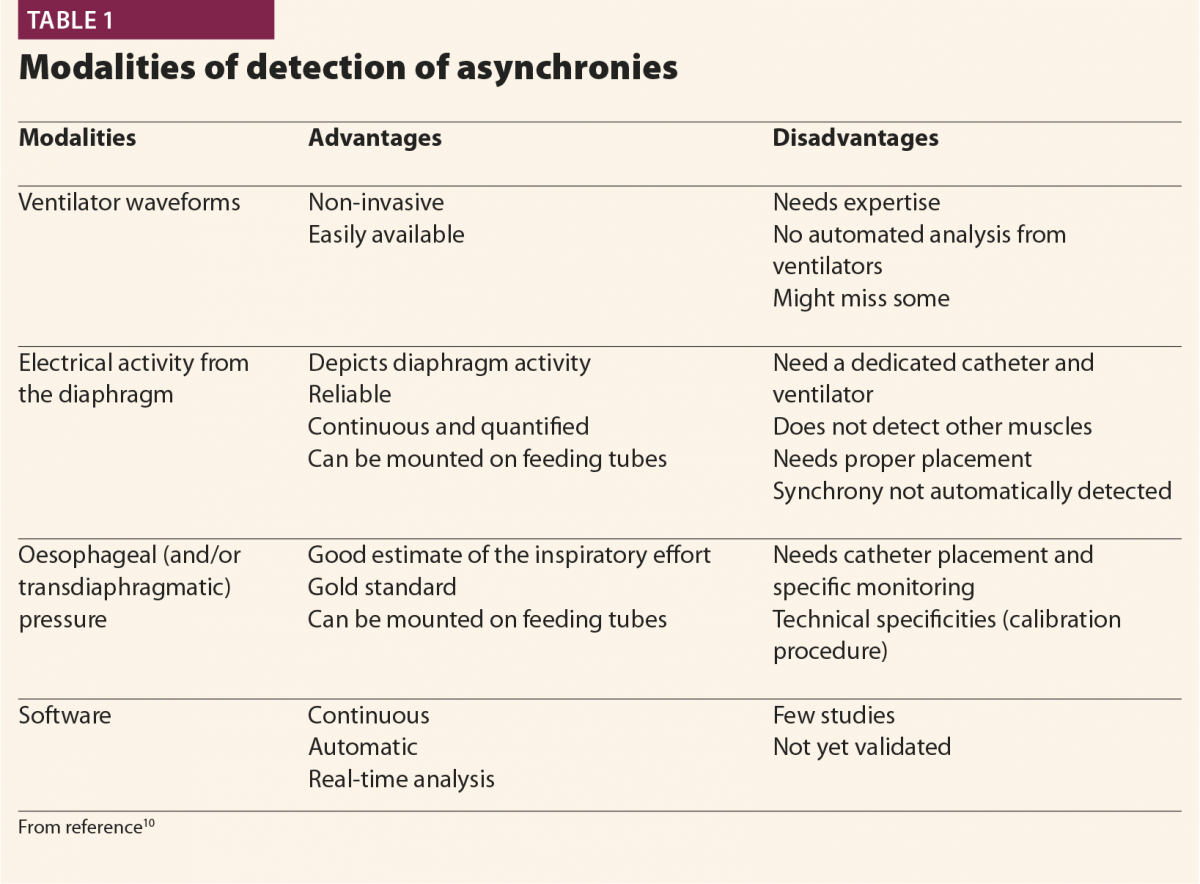
Clinicians progressively learnt how to visually detect asynchronies by looking at ventilator waveforms at the bedside and to adapt ventilator settings breath by breath accordingly, but also realised that the time required for such management was not compatible with everyday clinical practice in the ICU. In fact, patient–ventilator interaction is highly variable among different patients and, in the same patient at different times.4 Moreover, it was suggested that brief clusters of asynchronies and more than average frequency of asynchronies, are associated with poor outcome.19 But it is not feasible to stay at the bedside 24/7 to monitor asynchronies and change the ventilator’s setting according to waveforms. In this context, researchers and manufacturers put their efforts into developing new technologies that are able to replace clinicians in analysing ventilator waveforms and detecting patients’ respiratory activity.1
Detecting asynchronies at the bedside
One can approach asynchronies in two different ways: the first is to rely on software and technologies able to optimise the issue (mainly diaphragmatic electrical activity, other automatic triggering systems available on a few modern ICU ventilators). However, these tools are not always available for every ICU patient. The second approach is based on the observation of ventilator waveforms, the direct recognition of asynchronies and the optimisation of the ventilator settings. Obviously this method is more applicable in the ICU because it does not require special technologies; however, a good knowledge of the most frequent patterns, and of the underlying pathophysiology, is essential to make it efficient.
The waveform method is based on the observation of the standard curves displayed on the ventilator screen (flow and airway pressure), because they are as sensitive and specific as oesophageal pressure, which thus far is considered the gold standard for detection of asynchronies. The method is centred on the identification of the patient’s spontaneous activity.
Patient’s inspiration
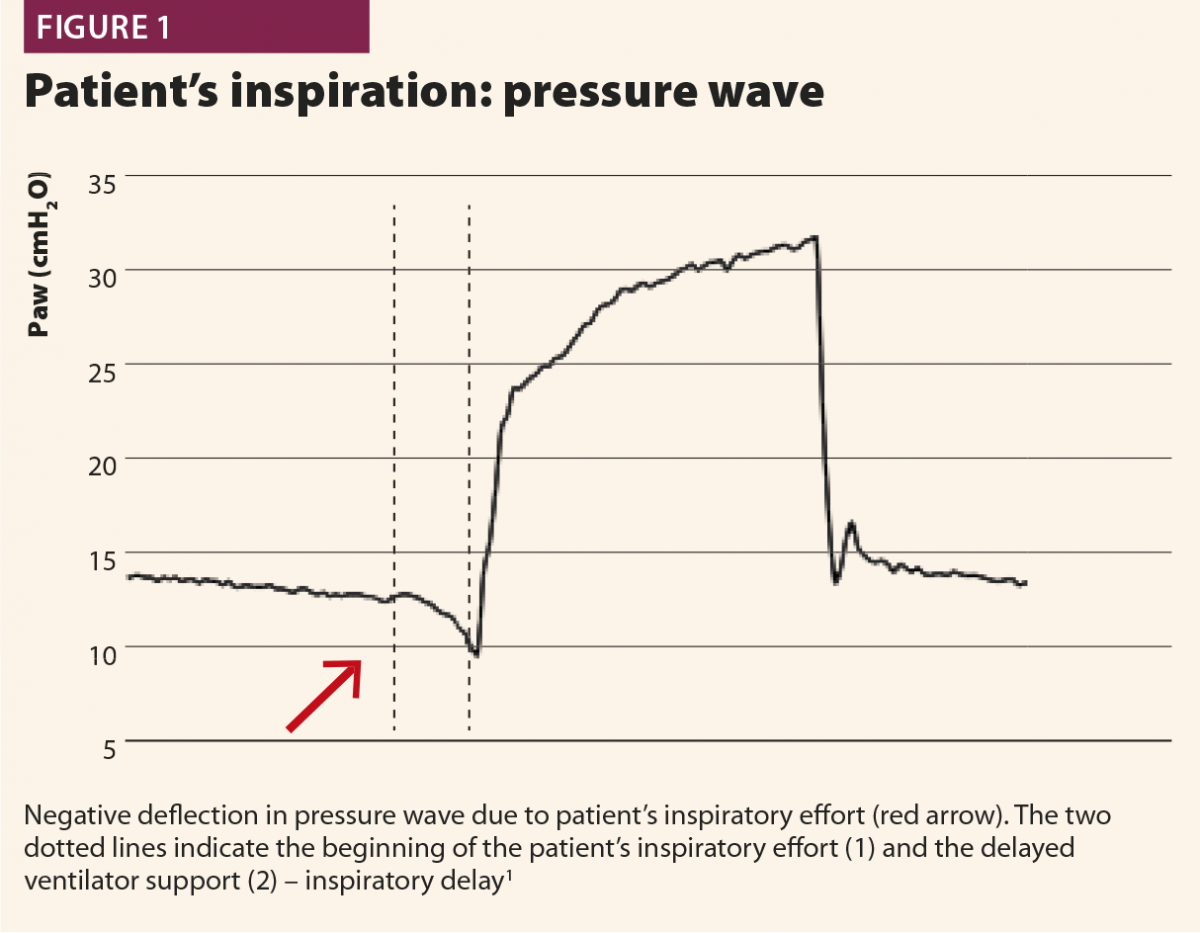
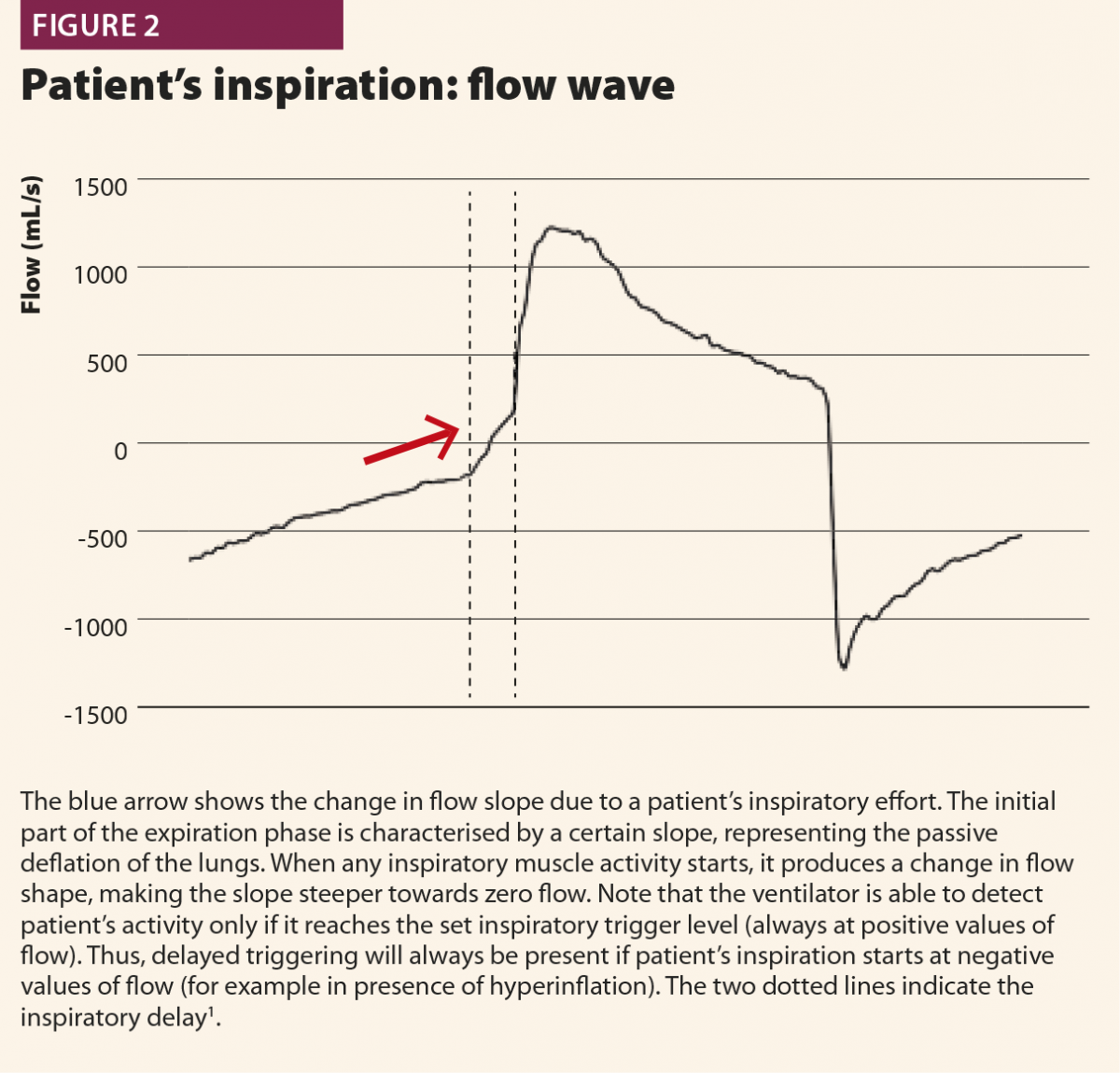
Typically, when a patient starts a breath, this causes a negative deflection on the pressure curve (Fig.1); on the flow curve, a positive deflection can be detected, even if flow is still negative (Fig.2). Flow and pressure changes correlate with oesophageal pressure, thus they are sufficient to detect a patient’s respiratory activity in most cases.6,7,13
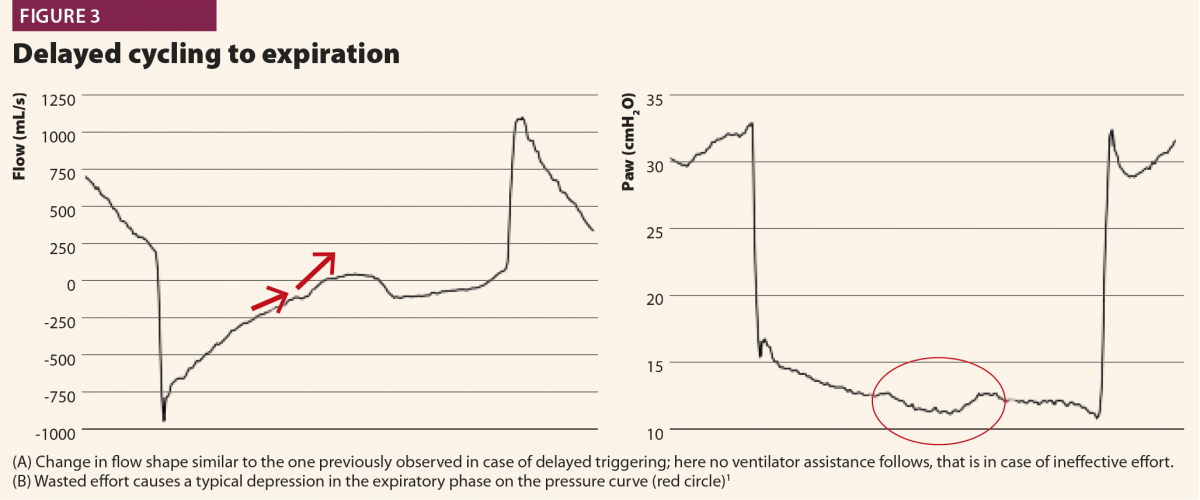
With these simple rules, a patient’s inspiratory activity can be detected even when it is neither detected nor assisted by the ventilator: in other words, ventilator waveforms can reveal a patient’s attempt to trigger the ventilator that does not reach its aim, namely an ineffective effort (Fig.3).
The patient’s start of expiration can also be detected on flow and pressure waves; physiologically, it corresponds to a time point between the nadir of muscular pressure curve and its return to the baseline. This time point varies from patient to patient depending on respiratory mechanics and breathing pattern, but can be conveniently approximated at half relaxation.8
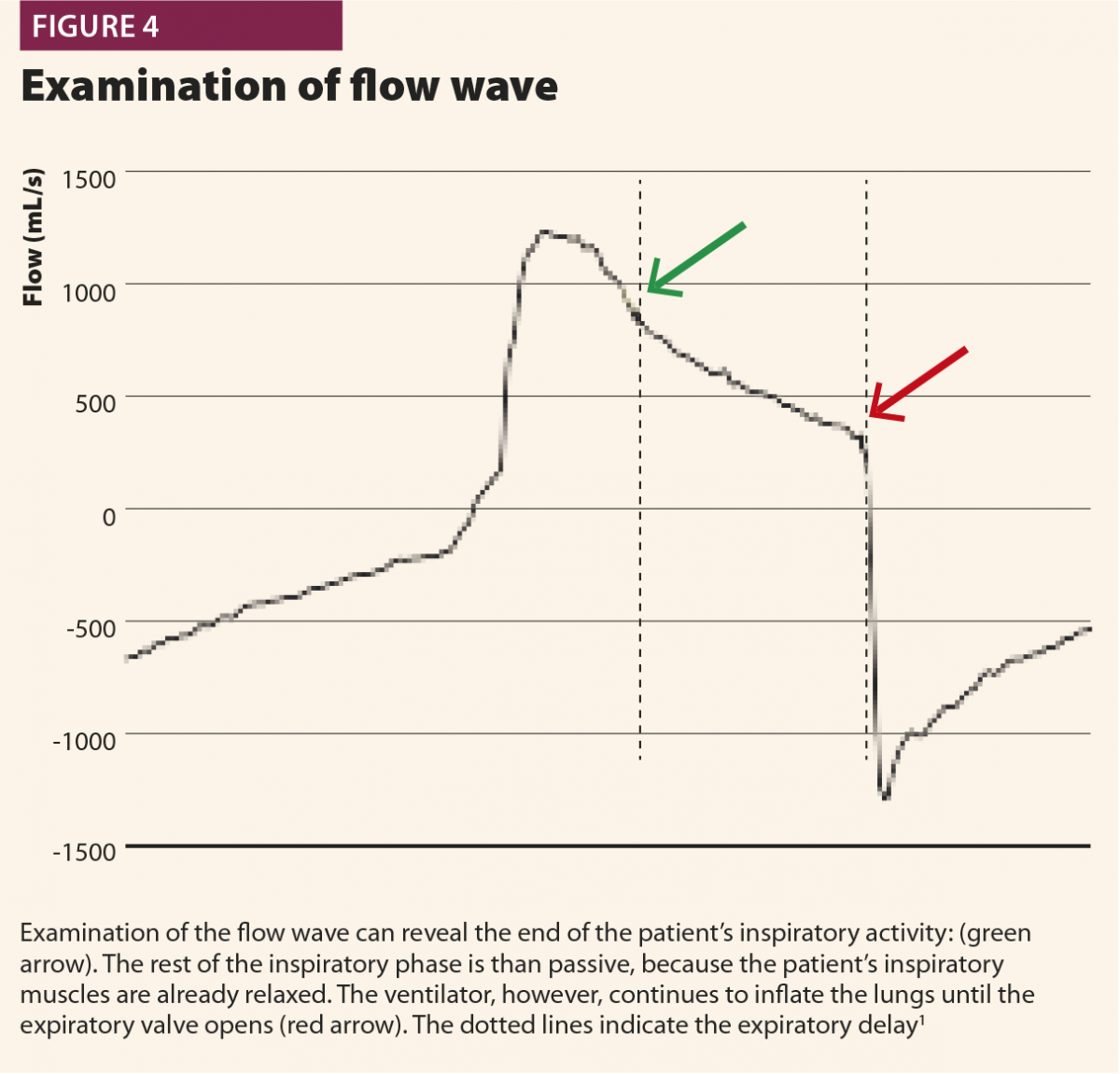
If a muscular pressure curve is not available, indirect signs of relaxation can be detected on flow wave and their appearance varies depending on the assistance given from the ventilator. There are three possible cases1: late cycling, early cycling and optimal cycling.
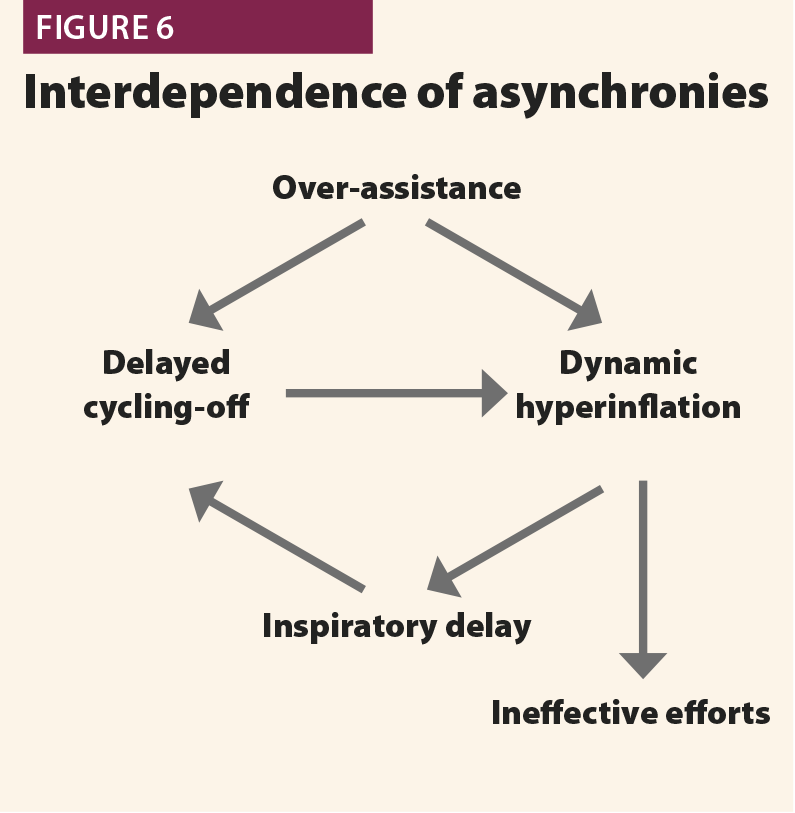
In the first, the machine aerogates air for longer than required; in this case, the patient’s inspiratory muscles will relax during the ventilator’s inspiratory phase, causing a sudden change from fast to slow decrease of inspiratory flow as shown in Fig.4. This often leads to hyperinflation, causing other asynchronies such as ineffective efforts and delayed triggering in the following breaths.20 This phenomenon (called late cycling) is typical of COPD patients and is promoted by a high level of pressure support. Sometimes, patients react to late cycling with active exhalation attemps while the ventilator’s inflation still ongoing, causing a positive deflection on the pressure wave.
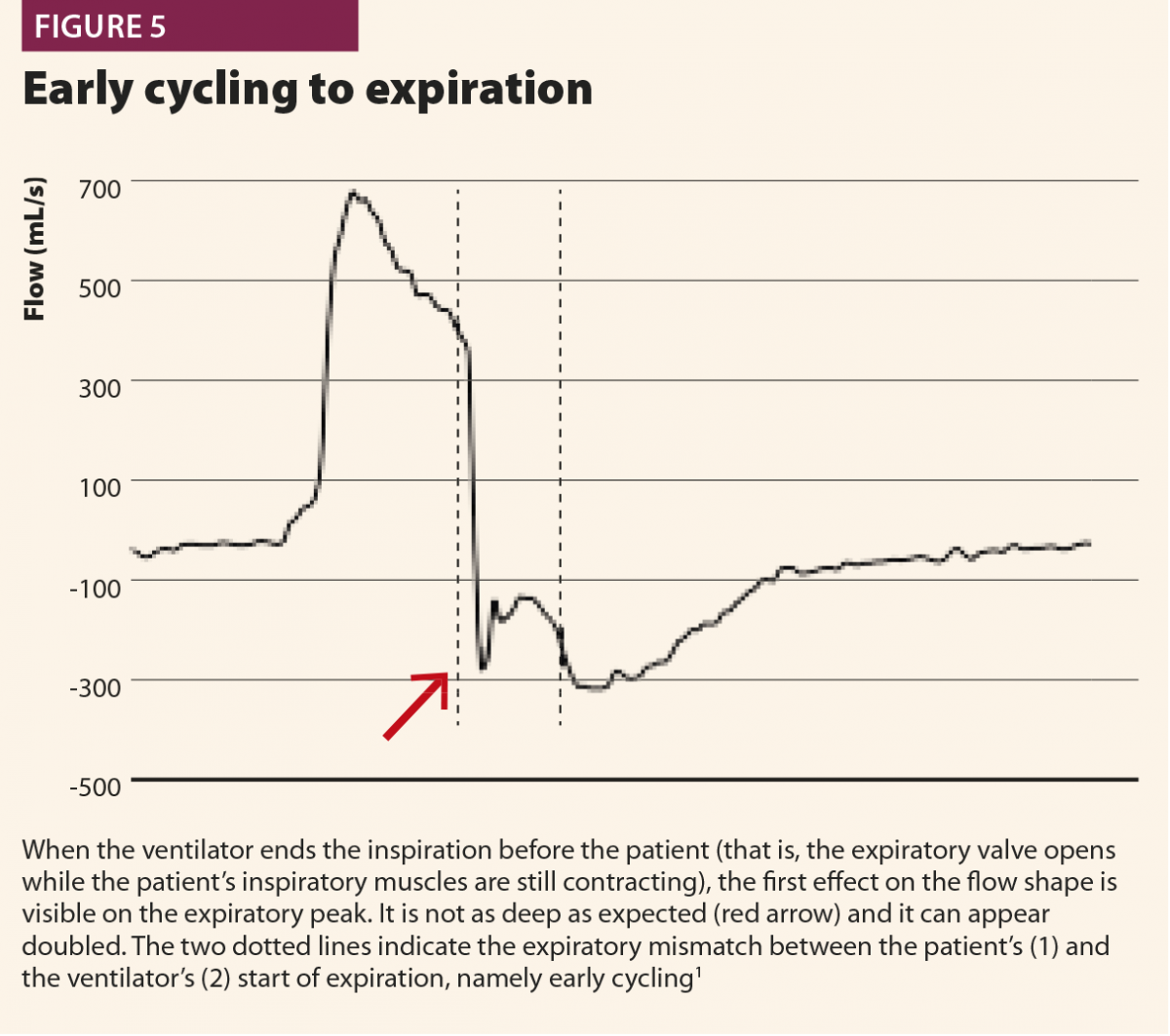
In a second possibility, the ventilator stops aerogating air when the patient’s muscles are still contracting, so expiratory flow is halted by the patient’s inspiratory activity extended after the opening of the expiratory valve, with a typical effect on expiratory peak flow, which appears cut, delayed or ‘doubled’ (Fig.5). Another possible consequence of early cycling to expiration is double triggering. Persistent patient activity after expiratory valve opening can again activate the trigger; thus the ventilator aerogates another breath immediately after the previous one, without a physiological exhalation in between.
In a third case, the ventilator ends its support exactly when the patient’s muscles relax: in this case, inspiratory flow decay becomes faster and faster, directly switching into expiratory flow, with immediate peak and then slow exponential decay.1
Bedside optimisation
Once the clinician has identified the patient’s activity and asynchronies observing the ventilator waveforms, there are a few interventions that can effectively solve the issue. Firstly, any source of external disturbance has to be eliminated (circuit leaks, secretions, circuit occlusions, deconnections), because they can lead to changes in the waveforms and thereby lead to misinterpretation. Second, clinicians have to be aware of the effects of a ventilator’s settings on asynchrony development and act on them appropriately to promote synchronisation.1
Inspiratory trigger
The appropriateness of the inspiratory trigger facilitates the breath initiation and decreases the patient’s work of breathing. Flow trigger is considered better than pressure trigger because it is more sensitive to a patient’s effort and does not require a negative pressure to be produced in the circuit to trigger the ventilator; a little flow entering the inspiratory valve is enough. This leads to more comfortable triggering; however, pressure triggers on modern ventilators have been improved, and the difference between flow and pressure triggers is often very fine.1 As a general rule, trigger sensitivity should be set at the highest value (lowest flow threshold) able to avoid autotriggers, in order to optimise the comfort of the patient.
Pressure support level
Overassistance facilitates asynchronies as well as muscle atrophy very high pressure support levels must be avoided. An excessive pressure support can worsen hyperinflation, leading to difficult triggering (trigger delay and ineffective efforts) and late cycling to expiration.21 When such asynchronies are detected on ventilator waveforms, physicians should consider a decrease of pressure support level.
Ramp
The ramp represents the flow speed to reach the inspiratory peak. As a general rule, for the same sensitivity of the expiratory trigger, a faster ramp makes cycling earlier, whereas a slower ramp makes cycling later. Therefore, a fast ramp can facilitate expiratory synchronisation, especially in COPD patients, whereas a slow ramp increases the time needed to reach lower peak inspiratory flow, thereby favouring late cycling to expiration.
Expiratory trigger sensitivity
The expiratory trigger sensitivity (ETS) is the percentage of the inspiratory flow peak that commands the expiratory valve opening and the cycling to expiration. It can be manually set from minimum values of 5% to maximum of 60–70% of the flow peak; default setting is usually 25% of flow peak.
Setting the ETS appropriately is essential for synchronisation.9,22,23 There is not a ‘one size fits all’ configuration: each patient needs a customised setting, based on the respiratory mechanics and the current respiratory pattern. If the ETS is too low, the ventilator will continue to inflate the patient’s lungs even after the respiratory muscles have relaxed. In other words, a certain amount of the inspiratory phase will be passive, without the participation of the patient’s muscles. By contrast, if the ETS is too high, the ventilator will stop aerogating air even if the respiratory muscles are still contracted: this ‘pliometric’ or ‘eccentric’ contraction can directly damage the diaphragm5,24,25 and can lead to double triggering, breath stacking and lung injury.
An optimised ETS can also positively affect the triggering phase, allowing a physiologic passive exhalation, minimising hyperinflation and ultimately facilitating the trigger for the following breath.
Because COPD patients are prone to late cycling, whereas restrictive patients can experience early cycling, a reasonable approach for initial ETS setting is 25% for patients with normal mechanics (RCexp 0.4–0.8 s), 10% for restrictive patients (RCexp <0.4 s) and 50% for COPD patients (RCexp >0.8 s). Thereafter, the interpretation of bedside ventilator waveforms can be used for fine tuning of ETS.
Sedation
Most of the patients ventilated in assisted modes require some sedation, at least for tube tolerance,26 but excessive sedation is associated with difficult ventilator triggering and with ineffective efforts, mainly for respiratory drive and muscular pressure reduction.15 Optimising sedation is mandatory for correct patient–ventilator interaction management: a lighter sedative plan promotes a patient’s own muscle activity and reduces asynchronies, also allowing
a reduction in pressure support levels.
Automatic monitoring
In the last ten years, big efforts have made in developing software able to detect patients’ respiratory activity and, by computing these data with the ventilator output, toidentify asynchronies. Most of these monitoring softwares were able to work online only for brief periods, usually from minutes to a few hours; in reality, they mainly worked as offline asynchrony analysers, particularly focused on major asynchronies.27–29 The only effective way to monitor patient–ventilator interaction online at the bedside is waveform analysis performed by the expert clinician: indeed, it allows the detection of asynchronies and concurrent optimisation of the ventilator settings. Inevitably, waveform analysis has specific requirements and costs. First, specific training is required, because general clinical expertise and experience do not necessarily correlate with the ability of clinicians in detecting asynchronies by waveform analysis.30–32 Moreover, performing waveform analysis at the bedside is time consuming and requires reoptimisation every time patients change their breathing pattern or their respiratory system resistance and/or compliance for any reason (bronchoconstriction, hyperinflation, increased or decreased pleural effusion…).
In this setting, there is a real clinical need for new technologies that can analyse ventilator waveforms automatically in real time (breath by breath) and continuously (24/7). The ideal software should be able to identify any patient’s respiratory activity, discriminating the beginning and the end of each inspiratory act; it should be able to work online as a trigger to command the inspiratory valve opening and closing according to the patient’s effort. Manufacturers have marketed systems that have been implemented into modern ICU ventilators. They are all promising tools, but not yet validated and no results are currently available to document their performance in improving patient–ventilator interaction.
Conclusions
It is beneficial for ventilated patients to be monitored and optimised in their interaction with the ventilator, and waveform analysis has become essential in administering high quality ventilation. Facing asynchronies requires good knowledge and specific training on the subject.
It also takes time to perform bedside waveform analysis, especially in those cases with difficult patient–ventilator interactions. A possible solution is automation and the market is introducing interesting technologies that will be able to replace the clinicians’ optimisation.
There is a need for further studies to evaluate the performance of new generation triggers in improving asynchronies; in the meantime, the waveform method and the bedside optimisation of ventilator settings remain the most efficient means to manage patient–ventilator interaction.
References
1 Orlando A. How to improve patient-ventilator synchrony. www.hamilton-medical.com/it/dam/jcr:72a82168-9d92-49a6-bb01-0f310dad8fbf… (accessed July 2019).
2 Epstein SK. How often does patient-ventilator asynchronies occur and what are the consequences? Resp Care 2011;56(1):25–38.
3 Sassoon CS. Triggering of the ventilator in patient-ventilator interactions. Resp Care 2011;56(1):39–51.
4 Blanch L et al. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med 2015;41(4):633–41.
5 Nilsestuen JO, Hargett KG. Using ventilator graphics to identify patient-ventilator asynchronies. Respir Care 2005;50(2):202–34; discussion 232–4.
6 Thille AW et al. Patient-ventilator asynchronies during assisted mechanical ventilation. Intensive Care Med 2006;32(10):1515–2.
7 Georgopoulos D, Prinianakis G, Kondili E. Bedside waveforms interpretation as a tool to identify patient-ventilator asynchronies. Intensive Care Med 2006;32:34–6.
8 Mojoli F et al. Is the ventilator switching from inspiration to expiration at the right time? Look at the waveforms! Intensive Care Med 2016;42(5):912–3.
9 Tassaux et al. Impact of expiratory trigger setting on delayed cycling and inspiratory muscles workload. Am J Resp Crit Care Med 2005;172(10):1283–9.
10 Dres M et al. Monitoring patient-ventilator asynchrony. Curr Opin Crit Care 2016;22:246–53.
11 Mojoli F et al. Continuous monitoring of patient-ventilator interaction in ICU patients undergoing prolonged mechanical ventilation. Intensive Care Med 2014;40.
12 Murias G et al. Patient-ventilator asynchrony. Curr Opin Crit Care 2016;22:53–9.
13 Chao D et al. Patient-ventilator trigger asynchrony in prolonged mechanical ventilation. Chest 1997;112:1152–9.
14 Kachmarek RM et al. Assisted mechanical ventilation: the future is now! BMC Anesthesiol 2015;15:110.
15 De Wit M et al. Observational study of patient-ventilator asynchrony and relation to sedation level. J Crit Care 2009;1:74–80.
16 Chanques G et al. Impact of ventilator adjustments and sedation-analgesia practices on severe asynchronies in patients ventilated in assist-control mode. Crit Care Med 2013;41(9):2177–87.
17 Evans KC et al. BOLD fMRI identifies limbic, paralimbic and cerebellar activation during air hunger. J Neurophysiol 2002;88(3):1500–11.
18 Huang M et al. Psychiatric symptoms in acute respiratory distress syndrome survivors: a one-year national multicenter study. Crit Care Med 2016;44(5):954–65.
19 Vaporidi K et al. Clusters of ineffective efforts during mechanical ventilation: impact on outcome. Intensive Care Med 2017;43(2):184–91.
20 Kachmarek et al. Cycle asynchrony: always a concern during pressure ventilation. Minerva Anestesiol 2016;82(7):728–30.
21 Thille AW et al. Reduction of patient-ventilator asynchrony by reducing tidal volume during pressure support ventilation. Intensive Care Med 2008;34(8):1477–86.
22 Chiumello D et al. Effects of different cycling-off criteria and positive end-expiratory pressure during pressure support ventilation in patients with chronic obstructive pulmonary disease. Crit Care Med 2007;35(11):2547–52.
23 Hoff FC et al. Cycling-off modes during pressure support ventilation: effects on breathing pattern, patient effort, and comfort. J Crit Care 2014;29(3):380–5.
24 Gea J et al. Modifications of diaphragm activity induced by midline laparotomy and changes in abdominal wall compliance. Arch Broncon 2009;45(1):30–5.
25 Devor ST et al. Regeneration of new fibers in muscles of old rats reduced contraction-induced injury. J Appl Phys 1999;87(2):750–6.
26 Vaschetto R et al. Effects of propofol on patient-ventilator synchrony and interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med 2014;42(1):74–82.
27 Younes M et al. A method for improving patient-ventilator interaction. Intensive Care Med 2007;33:1337–46.
28 Blanch L et al. Validation of the Better Care System to detect ineffective efforts during expiration in mechanically ventilated patients: a pilot study. Intensive Care Med 2012;38(2):240–7.
29 Sinderby C et al. An automated and standardized neural index to quantify patient-ventilator interaction. Crit Care 2013;17:R239.
30 Colombo D et al. Efficacy of ventilator waveforms observation in detecting patient-ventilator asynchrony. Crit Care Med 2011;39(11):2452–7.
31 Ramirez I et al. Ability of health care professionals to identify patient-ventilator asynchrony using waveform analysis. Resp Care 2017;62(2):144–9.
32 Prinianakis G et al. Effect of the flow waveform method of triggering and cycling on patient-ventilator interaction during pressure support. Intensive Care Med 2003;29(11):1950–9










